
The association between robotic lung cancer resection and esophagectomy outcomes: a facility-level analysis
Highlight box
Key findings
• Conversion from robotic to open esophagectomy occurs more frequently at centers performing a low volume of robotic lung resections, when compared to facilities performing a high volume of robotic lung resections.
What is known and what is new?
• Unplanned conversion from minimally invasive to open procedures can result in patient morbidity and/or mortality.
• Facility-level robotic lung resection surgical volume may influence robotic esophagectomy conversion rates.
What is the implication and what should change now?
• Robotic operative experience might be transferable between procedures. Defining facility volume minimums for robotic procedures and exploring the relationship between overall surgical volume, approach-specific surgical volume and patient outcomes is worthy of further study.
Introduction
Background
Robot-assisted thoracoscopic surgery (RVATS) has grown dramatically in popularity over the past decade, and is increasingly the approach of choice for lung resection (1). There are several advantages associated with robotic approach, including enhanced 3D visualization and improved dexterity (2). RVATS has historically been viewed as comparable to video-assisted thoracoscopic surgery (VATS) with respect to complication rates and survival, though some data suggests it is favorable with respect to 30-day mortality and post-operative quality of life (3,4). Similarly, robotic-assisted minimally invasive esophagectomy (RAMIE) has been shown to be at least non-inferior to minimally-invasive esophagectomy, and may offer advantages with respect to lymph node harvest and probability of conversion to open (5,6). However, robotic surgery requires significant investment at an institutional and individual level (7). The devices and accessories are costly and implementing a successful robotic surgery program requires extensive training for operationalization (8). There is a steep learning curve associated with robotic surgery, especially complex procedures such as RAMIE (9).
Rationale and knowledge gap
A risk of any minimally invasive surgery is urgent or emergent conversion to an open procedure. This risk is heightened when surgeons are gaining familiarity with a surgical platform (9). Conversion from RVATS or RAMIE to an open procedure is associated with higher complication rates, longer length of stay, and higher 30- & 90-day mortality (10-13). Patient-specific factors such as age, squamous cell histology and Charlson-Deyo Comorbidity Index (CCI) have been associated with increased risk of conversion (12). Additionally, a recent analysis by Servais et al. (10) determined that annual center volume was a significant predictor for conversion from RVATS to open thoracotomy, an impact that was most pronounced at low volume centers.
Objective
As surgeons have greater access to robotic systems and become facile with the technology, it is unclear if skills honed on other robotic procedures impact surgeon performance and patient outcomes for more complex robotic procedures. We aim to study this question within the context of thoracic surgery. This paper examines the association between facility-level robotic-assisted lung cancer resection and RAMIE surgical volumes, and rates of conversion to open procedures. We present this article in accordance with the STROBE reporting checklist (available at https://vats.amegroups.com/article/view/10.21037/vats-23-47/rc).
Methods
Data source and patient selection
Data was extracted from the National Cancer Database (NCDB), which aggregates hospital registry data for clinical oncology patients. The NCDB includes data from over 1,500 Commission on Cancer (CoC) facilities over a longitudinal time-period. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This project was deemed exempt from ethics committee approval and informed consent by the Institutional Review Board at Thomas Jefferson University Hospital.
Individual facilities were analyzed by year. Facility-years between 2010 and 2018 with at least one robotic lung resection for cancer and one RAMIE were included in the analysis. Facility-years were divided into low-volume or high-volume centers based on the number of robotic lung cancer resections performed annually and number of RAMIEs performed annually. At time of analysis, 2018 was the latest year of data available. A cutoff of fewer than nine RAMIEs/annum was chosen to designate a low volume center based on prior analysis by Hue et al. (14), whose work showed that centers performing fewer than nine RAMIEs/year had higher mortality rates and more complications than those performing greater than nine RAMIEs yearly.
A cutoff of 20 robotic lung cancer resections per year was chosen to designate a high-volume center for robotic lung surgery. The Leapfrog minimum volume cutoff to designate a high-volume center is 40 lobectomies per year but this figure does not take into account surgical approach. Little data exists regarding approach-specific minimum volume cutoffs, and existing studies from a similar time period and the same database indicate that a minimally invasive approach was chosen less than 50% of the time (12,15). This was confirmed by our initial review of the data, which showed that very few centers performed 40 or more robotic lung cancer resections annually. Thus, a cutoff of 20 robotic lung resections annually was chosen to indicate a high-volume facility, with the reasonable assumption that those facilities performed additional open or VATS procedures, and overall performed more surgery than what has in the past been referred to as low volume centers.
Patients aged eighteen or older who received either a robotic lung resection or a robotic esophagectomy within the included facility-years were analyzed. The designation of approach type (open, laparoscopic/thoracoscopic or robotic) is coded at the facility level and may include cases with a robotic component and a planned open, laparoscopic or thoracoscopic component.
For the purposes of our study, wedge resections, segmentectomy, lobectomy, or pneumonectomy performed on individuals with a diagnosis of lung cancer were included. Facility-years during which no robotic lung cancer resections or esophagectomies were performed were excluded from the analysis. Facility-years were categorized into one of four groups: low volume robotic lung, low volume robotic esophagectomy (LL, LE); high volume robotic lung, low volume robotic esophagectomy (HL, LE); low volume robotic lung, high volume robotic esophagectomy (LL, HE); high volume robotic lung, high volume robotic esophagectomy (HL, HE).
Variables
Baseline patient characteristics were collected including year of diagnosis, age, sex, race/ethnicity, insurance status, median income, CCI, and both 30- and 90-day mortality. Hospital level factors assessed included rates of robotic lung cancer resection conversion, rates of robotic esophagectomy conversion, facility type, geography, and location. Robotic conversion rates were calculated by dividing the number of cases in which the robotic attempt was converted to an open procedure by the total number of robotic cases (including those converted to open) for the given facility-year. No information is available regarding individual surgeon volume is available from the NCDB, nor information regarding why cases were converted to open. Additionally, information on post-operative complication rates and types are not available from the NCDB.
Statistical analysis
Student’s t-tests were utilized to compare patient characteristics. All data was examined using STATA/SE 17.0 statistical software (StataCorp LLC, College Station, TX, USA) with a 2-sided significance level of P<0.05.
Results
Patient characteristics
A total of 16,178 patients underwent robotic lung cancer resections during the included facility-years. Patients who underwent lung resection had a median age of 68 years [interquartile range (IQR), 62–74 years], and the majority were female (9,269; 57.3%), White (13,203; 81.6%), and on Medicare insurance (10,268; 63.6%). Additional demographic data is listed in Table 1.
Table 1
| Variable | Total robotic lung resections (N=16,178) | LL, LE (N=2,487) |
HL, LE (N=11,069) |
LL, HE (N=148) |
HL, HE (N=2,474) | P value |
|---|---|---|---|---|---|---|
| Year of diagnosis, n (%) | <0.001 | |||||
| 2010 | 299 (1.8) | 98 (3.9) | 156 (1.4) | 0 (0.0) | 45 (1.8) | |
| 2011 | 573 (3.5) | 139 (5.6) | 324 (2.9) | 0 (0.0) | 110 (4.4) | |
| 2012 | 981 (6.1) | 214 (8.6) | 550 (5.0) | 19 (12.8) | 198 (8.0) | |
| 2013 | 1,074 (6.6) | 236 (9.5) | 629 (5.7) | 29 (19.6) | 180 (7.3) | |
| 2014 | 1,271 (7.9) | 188 (7.6) | 870 (7.9) | 12 (8.1) | 201 (8.1) | |
| 2015 | 1,564 (9.7) | 295 (11.9) | 912 (8.2) | 15 (10.1) | 342 (13.8) | |
| 2016 | 2,213 (13.7) | 387 (15.6) | 1,629 (14.7) | 17 (11.5) | 180 (7.3) | |
| 2017 | 3,443 (21.3) | 514 (20.7) | 2,373 (21.4) | 21 (14.2) | 535 (21.6) | |
| 2018 | 4,760 (29.4) | 416 (16.7) | 3,626 (32.8) | 35 (23.6) | 683 (27.6) | |
| Age at diagnosis, years, median [IQR] | 68 [62–74] | 68 [61–74] | 68 [61–74] | 69 [61–76] | 69 [62–75] | 0.01 |
| Sex, n (%) | 0.82 | |||||
| Male | 6,909 (42.7) | 1,067 (42.9) | 4,709 (42.5) | 68 (45.9) | 1,065 (43.0) | |
| Female | 9,269 (57.3) | 1,420 (57.1) | 6,360 (57.5) | 80 (54.1) | 1,409 (57.0) | |
| Race/ethnicity, n (%) | <0.001 | |||||
| White | 13,203 (81.6) | 2,024 (81.4) | 8,969 (81.0) | 117 (79.1) | 2,093 (84.6) | |
| Black | 1,437 (8.9) | 255 (10.3) | 977 (8.8) | 26 (17.6) | 179 (7.2) | |
| Hispanic | 727 (4.5) | 82 (3.3) | 569 (5.1) | 0 (0.0) | 76 (3.1) | |
| API | 597 (3.7) | 92 (3.7) | 414 (3.7) | 4 (2.7) | 87 (3.5) | |
| Other | 214 (1.3) | 34 (1.4) | 140 (1.3) | 1 (0.7) | 39 (1.6) | |
| Insurance, n (%) | <0.001 | |||||
| Private | 4,547 (28.1) | 756 (30.8) | 3,087 (27.9) | 42 (28.4) | 662 (26.9) | |
| Medicare | 10,286 (63.6) | 1,513 (61.6) | 7,074 (63.9) | 100 (67.6) | 1,599 (65.1) | |
| None/other/no data | 1,345 (8.3) | 188 (7.7) | 908 (8.2) | 6 (4.1) | 196 (8.0) | |
| Median income (USD), n (%) | <0.001 | |||||
| <$38,000 | 2,256 (16.6) | 336 (16.0) | 1,495 (13.5) | 27 (18.2) | 398 (16.1) | |
| $38,000–$47,999 | 3,002 (22.1) | 448 (21.4) | 1,895 (17.1) | 35 (23.7) | 624 (25.2) | |
| $48,000–$62,999 | 3,608 (26.5) | 537 (25.6) | 2,393 (21.6) | 47 (31.8) | 631 (25.5) | |
| ≥$63,000 | 4,732 (34.8) | 776 (37.0) | 3,383 (30.6) | 31 (20.9) | 542 (21.9) | |
| No data | 0 (0) | 0 (0) | 1,903 (17.2) | <10 (5.4) | 279 (11.3) | |
| CCI, n (%) | 0.018 | |||||
| 0 | 8,870 (54.8) | 1,330 (53.5) | 6,064 (54.8) | 69 (46.6) | 1,407 (56.9) | |
| ≥1 | 7,308 (45.2) | 1,157 (46.5) | 5,005 (45.2) | 79 (53.4) | 1,067 (43.1) | |
| Facility type, n (%) | <0.001 | |||||
| Academic | 9,405 (58.1) | 1,055 (42.4) | 6,364 (57.5) | 128 (86.5) | 1,858 (75.1) | |
| Community | 91 (0.6) | 61 (2.5) | 21 (0.2) | 9 (6.1) | 0 (0.0) | |
| Comprehensive | 2,698 (16.7) | 796 (32.0) | 1,680 (15.2) | 0 (0.0) | 222 (9.0) | |
| Integrated | 3,859 (23.9) | 556 (22.4) | 2,916 (26.3) | 11 (7.4) | 376 (15.2) | |
| Other | 125 (0.8) | 19 (0.8) | 88 (0.8) | 0 (0.0) | 18 (0.7) | |
| Facility geography, n (%) | <0.001 | |||||
| Metro | 13,866 (85.7) | 2,001 (83.8) | 9,531 (86.1) | 117 (79.1) | 2,217 (89.6) | |
| Urban | 1,548 (9.6) | 352 (14.7) | 1,033 (9.3) | 20 (13.5) | 143 (5.8) | |
| Rural | 115 (0.7) | 34 (1.4) | 62 (0.6) | <10 (1.4) | 17 (0.7) | |
| No data | 649 (4.0) | 0 (0) | 443 (4.0) | <10 (6.1) | 97 (3.9) |
LL, low robotic lung resection volume; HL, high robotic lung resection volume; LE, low RAMIE volume; HE, high RAMIE volume; IQR, interquartile range; API, Asian/Pacific Islander; CCI, Charlson-Deyo Comorbidity Index; RAMIE, robotic-assisted minimally invasive esophagectomy.
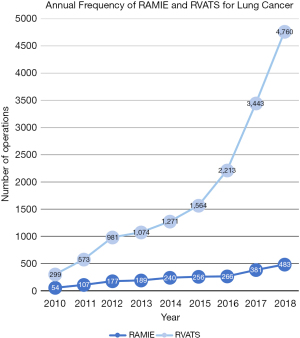
A total of 2,153 patients received robotic esophagectomies during the included facility-years (Table 2). Patients who underwent robotic esophagectomy had a median age of 65 years (IQR, 58–71 years), and the majority were male (1,797; 83.5%), White (1,901; 88.3%), and on Medicare insurance (49.3%). The overall number of robotic surgical procedures increased annually (Figure 1).
Table 2
| Variable | Total RAMIEs (N=2,153) |
LL, LE (N=687) |
HL, LE (N=698) |
LL, HE (N=200) |
HL, HE (N=568) |
P value |
|---|---|---|---|---|---|---|
| Year of diagnosis, n (%) | <0.001 | |||||
| 2010 | 54 (2.5) | 29 (4.2) | 8 (1.1) | 0 (0.0) | 17 (3.0) | |
| 2011 | 107 (5.0) | 43 (6.3) | 30 (4.3) | 0 (0.0) | 34 (6.0) | |
| 2012 | 177 (8.2) | 68 (9.9) | 36 (5.2) | 23 (11.5) | 50 (8.8) | |
| 2013 | 189 (8.8) | 58 (8.4) | 56 (8.0) | 28 (14.0) | 47 (8.3) | |
| 2014 | 240 (11.1) | 67 (9.8) | 67 (9.6) | 40 (20.0) | 66 (11.6) | |
| 2015 | 256 (11.9) | 84 (12.2) | 48 (6.9) | 28 (14.0) | 96 (16.9) | |
| 2016 | 266 (12.4) | 99 (14.4) | 101 (14.5) | 26 (13.0) | 40 (7.0) | |
| 2017 | 381 (17.7) | 136 (19.8) | 134 (19.2) | 19 (9.5) | 92 (16.2) | |
| 2018 | 483 (22.4) | 103 (15.0) | 218 (31.2) | 36 (18.0) | 126 (22.2) | |
| Age at diagnosis, years, median [IQR] | 65 [58–71] | 65 [58–70] | 65 [57–71] | 64 [58–71] | 64 [57–71] | 0.93 |
| Sex, n (%) | 0.55 | |||||
| Male | 1,797 (83.5) | 579 (84.3) | 572 (81.9) | 171 (85.5) | 475 (83.6) | |
| Female | 356 (16.5) | 108 (15.7) | 126 (18.1) | 29 (14.5) | 93 (16.4) | |
| Race/ethnicity, n (%) | 0.002 | |||||
| White | 1,901 (88.3) | 599 (87.2) | 595 (85.2) | 188 (94.0) | 519 (91.4) | |
| Black | 88 (4.1) | 29 (4.2) | 37 (5.3) | 9 (4.5) | 13 (2.3) | |
| Hispanic | 82 (3.8) | 26 (3.8) | 38 (5.4) | 1 (0.5) | 17 (3.0) | |
| API | 53 (2.5) | 21 (3.1) | 21 (3.0) | 2 (1.0) | 9 (1.6) | |
| Other | 29 (1.3) | 12 (1.7) | 7 (1.0) | 0 (0.0) | 10 (1.8) | |
| Insurance, n (%) | 0.77 | |||||
| Private | 893 (41.9) | 284 (41.3) | 290 (41.5) | 76 (38.0) | 243 (42.8) | |
| Medicare | 1,051 (49.3) | 336 (48.9) | 336 (48.1) | 108 (54.0) | 271 (47.7) | |
| None/other/no data | 189 (8.9) | 67 (9.8) | 72 (10.3) | 16 (8.0) | 54 (9.5) | |
| Median income (USD), n (%) | 0.003 | |||||
| <$38,000 | 262 (12.2) | 87 (12.7) | 78 (11.2) | 20 (10.0) | 77 (13.6) | |
| $38,000–$47,999 | 416 (19.3) | 139 (20.2) | 109 (15.6) | 47 (23.5) | 121 (21.3) | |
| $48,000–$62,999 | 534 (24.8) | 149 (21.7) | 175 (25.1) | 67 (33.5) | 143 (25.2) | |
| ≥$63,000 | 619 (28.8) | 217 (31.6) | 214 (30.7) | 54 (27.0) | 134 (23.6) | |
| No data | 322 (15.0) | 95 (13.8) | 122 (17.5) | 12 (6.0) | 93 (16.4) | |
| CCI, n (%) | 0.072 | |||||
| 0 | 1,488 (69.1) | 490 (71.3) | 482 (69.1) | 123 (61.5) | 393 (69.2) | |
| ≥1 | 665 (30.9) | 197 (28.7) | 216 (30.9) | 77 (38.5) | 175 (30.8) | |
| Facility type, n (%) | <0.001 | |||||
| Academic | 1,337 (62.1) | 321 (46.7) | 431 (61.7) | 126 (63.0) | 459 (80.8) | |
| Community | 33 (1.5) | 8 (1.2) | 1 (0.1) | 24 (12.0) | 0 (0.0) | |
| Comprehensive | 319 (14.8) | 173 (25.2) | 112 (16.0) | 0 (0.0) | 34 (6.0) | |
| Integrated | 437 (20.3) | 176 (25.6) | 146 (20.9) | 48 (24.0) | 67 (11.8) | |
| Other | 27 (1.3) | 9 (1.3) | 8 (1.1) | 2 (1.0) | 8 (1.4) | |
| Facility geography, n (%) | <0.001 | |||||
| Metro | 1,788 (83.0) | 550 (80.0) | 597 (85.5) | 140 (70.0) | 501 (88.2) | |
| Urban | 220 (10.2) | 96 (14.0) | 60 (8.6) | 29 (14.5) | 35 (6.2) | |
| Rural | 17 (0.8) | <10 (1.2) | <10 (0.7) | <10 (1.0) | <10 (0.4) | |
| No data | 128 (5.9) | 33 (4.8) | 36 (5.2) | 29 (14.5) | 30 (5.3) |
RAMIE, robotic-assisted minimally invasive esophagectomy; LL, low robotic lung resection volume; HL, high robotic lung resection volume; LE, low RAMIE volume; HE, high RAMIE volume; IQR, interquartile range; API, Asian/Pacific Islander; CCI, Charlson-Deyo Comorbidity Index.
Robotic conversion rates—RAMIE
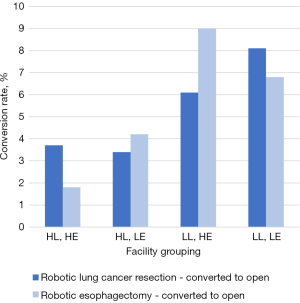
Over the period of this study [2010–2018], a total of 640 facility-years were included in the analysis and divided into four respective groups by volume (Table 3). The robotic esophagectomy conversion rate was highest at facilities with a low volume of robotic lung resections, and a high volume of RAMIEs (LL, HE group—18 facilities; 9.0% conversion rate) and lowest at facilities performing a high volume of both robotic lung resections and RAMIEs (HL, HE group—10 facilities; 1.8% conversion rate) (P<0.001).
Table 3
| Variable | LL, LE | HL, LE | LL, HE | HL, HE | P value |
|---|---|---|---|---|---|
| Total facility years | 340 | 243 | 19 | 38 | |
| Robotic lung cancer resection | 2,487 | 11,069 | 148 | 2,474 | |
| No conversion | 2,285 (91.9) | 10,698 (96.6) | 139 (93.9) | 2,382 (96.3) | <0.001 |
| Convert to open | 202 (8.1) | 371 (3.4) | 9 (6.1) | 92 (3.7) | |
| RAMIE | 687 | 698 | 200 | 568 | |
| No conversion | 640 (93.2) | 669 (95.8) | 182 (91.0) | 558 (98.2) | <0.001 |
| Convert to open | 47 (6.8) | 29 (4.2) | 18 (9.0) | 10 (1.8) |
LL, low robotic lung resection volume; HL, high robotic lung resection volume; LE, low RAMIE volume; HE, high RAMIE volume; RAMIE, robotic-assisted minimally invasive esophagectomy.
At low volume RAMIE facilities, conversion was more common if those facilities also performed a low volume of robotic lung resections (LL, LE: 47 facilities; 6.8% conversion rate), when compared to low volume RAMIE/high volume robotic lung resection facilities (HL, LE: 29 facilities; 4.2% conversion rate) (P<0.001).
Robotic conversion rates—robotic lung resection
Conversion rates for robotic lung cancer resections were higher at low-volume centers [LL, LE: 202 (8.1%); LL, HE: 9 (6.1%); P<0.001] than high-volume centers. The robotic lung cancer resection conversion rate was similar at high volume centers, irrespective of RAMIE surgical volume [HL, LE: 371 (3.4%); HL, HE: 92 (3.7%); P<0.001].
The 30- and 90-day mortality
The 30-day mortality was higher for robotic lung cancer resection patients whose procedures were converted to open when compared to those whose were not (5.8% vs. 1.1%, P<0.001). The same is true for 90-day mortality (9.1% vs. 2.1%, P<0.001). The 30-day mortality was similar for robotic esophagectomy patients whose procedures were converted to open compared to those whose were not (4.8% vs. 3.6%, P=0.53). The 90-day mortality was higher for patients whose procedures were converted to open compared to those whose were not converted, but not to a statistically significant degree (11.5% vs. 7.2%, P=0.097) (Table 4). Readmission rates were not statistically significant between groups.
Table 4
| Mortality | Robotic lung cancer resection | RAMIE | |||||
|---|---|---|---|---|---|---|---|
| Robot assist | Convert to open | P value | Robot assist | Convert to open | P value | ||
| 30-day | <0.001 | 0.53 | |||||
| Alive | 15,336 (98.9) | 635 (94.2) | 1,975 (96.4) | 99 (95.2) | |||
| Died | 168 (1.1) | 39 (5.8) | 74 (3.6) | 5 (4.8) | |||
| 90-day | <0.001 | 0.097 | |||||
| Alive | 15,182 (97.9) | 613 (90.9) | 1,902 (92.8) | 92 (88.5) | |||
| Died | 322 (2.1) | 61 (9.1) | 147 (7.2) | 12 (11.5) | |||
RAMIE, robotic-assisted minimally invasive esophagectomy.
Discussion
Key findings
Unplanned conversion from minimally invasive procedures to open has been well-documented to have a negative impact on patient outcomes, including increased postoperative morbidity and mortality, as well as longer length of stay (11,13,16). At centers performing a low volume of robotic lung cancer resections, conversion from RAMIE to open esophagectomy occurred at significantly higher rates than at centers performing a high volume of robotic lung cancer resections. The highest conversion rates occurred at centers that performed a high volume of esophagectomies but a low volume of robotic lung cancer resections (Figure 2). This finding suggests that facility-level robotic lung resection surgical volume may influence RAMIE conversion rate, with the strongest effect seen at high volume robotic esophagectomy facilities. RAMIE surgical volume does not appear to have a strong association with robotic lung cancer resection conversion rates. While we found that among patients undergoing robotic lung cancer resections, 30- and 90-day mortality were higher for those whose procedures were converted to open, we did not find this to be the case for converted RAMIEs. This is contrary to existing research (5,13), and could be secondary to small sample size in our study or additional variables not captured by the NCDB.
Comparison with similar research
The impact of surgical volume on patient outcomes has been studied extensively. Receipt of surgery at high-volume centers is associated with decreased operative mortality and length of stay (9,17-20). This finding has driven the creation of minimum-volume thresholds, as well as calls for centralization of care for complex, high-risk procedures such as esophagectomy (17,18,21). However, minimum-volume thresholds for robotic procedures have not been defined. While facility RVATS volume has been shown to be a predictor of conversion (10), the relationship between overall surgical volume, approach-specific surgical volume (thoracoscopic, robotic, and open) and patient outcomes remains underexplored. This is likely at least in part due to the rapid uptake of robotic surgical platforms over the past decade, and incomplete maturation of that data. While surgeons should strive to be facile in all approaches, robotic surgery performed at a center which is high volume for open surgery, and categorized as such by Leapfrog metrics, but low volume for robotic surgery may result in suboptimal patient outcomes, especially if the operating surgeon is inexperienced on the platform (9).
Risk factors for conversion from VATS to open thoracotomy have been assessed (5,10,16) but only a few studies have looked specifically at factors influencing conversion rates from RVATS to open thoracotomy. The available data suggests that overall, RVATS confers a lower risk of conversion to open than VATS (10,22,23). Identified risk factors for conversion for both RVATS and VATS include tumor size, preoperative radiation therapy, and facility surgical volume (16,23). Additional risk factors associated with conversion to open from VATS, such as obesity and preoperative chemotherapy, appear to have a minimal impact on conversion from RVATS to open (10). The reasons for conversion also differ between VATS and RVATS: the most common cause of RVATS conversion is major bleeding, whereas for VATS anatomic considerations (adhesions, difficult lymph node retrieval, etc.) appear to significantly influence conversion rate (10).
While data is limited with respect to conversion from RAMIE to open, available literature suggests that similar to RVATS, conversion from RAMIE to open occurs less frequently than conversion from minimally invasive (thoracoscopic/laparoscopic) esophagectomy to open (5,23,24). Identified risk factors for conversion from RAMIE to open include NSQIP estimated surgical risk score, and squamous cell histology (5,13).
Explanation of findings
That the literature shows lower conversion rates for RVATS/RAMIE, and worse outcomes for patients whose procedures were converted, additional metrics to appropriately risk stratify patients and minimize probability of conversion is important. This is especially salient given the growing popularity of the robotic approach. While historically patient selection may have biased preferable outcomes in robotic surgery, as the platform becomes the default approach, these factors may hold less weight. The preponderance of cases performed by robotic thoracic surgeons continue to be lung resections (1). As such, many of the robotic skills of the surgeon and the comfort level of the team are built around these cases. RAMIE remains an infrequently performed procedure usually limited to specialty centers (7). Thus, a reasonable assertion can be made that for thoracic surgeons, the overall robotic skill set necessary to complete these procedures is founded on robotic pulmonary resection, and lung resection experience sets the floor for overall thoracic robotic skill.
Implications and actions needed
Our data suggests that for robotic thoracic surgery, facility volume for some procedures may help predict risk of conversion for others and could conceivably be used as a component of risk stratification. To our knowledge, this is the first analysis of this nature. Further study is needed to explore additional correlative and causative factors, as well as to define quality benchmarks for facility volume for robotic procedures.
Limitations
There are several limitations to this study. Most importantly, this is a retrospective study using a large national database, and so selection biases may be present. Extraction of this data relies on proper data entry, so coding errors in the source data may be present. Though 640 facility-years are included in this analysis, a relatively low number of cases overall were converted to open, especially at high volume centers, and though a formal analysis was not performed, the data may be underpowered. This is particularly relevant with respect to esophagectomy data. While robotic lung cancer resection surgical volume appears to influence rates of RAMIE conversion at low volume robotic lung resection centers, causality cannot be determined. Additionally, there are certain elements that cannot be elucidated from the source data. For example, there is no way to determine how surgeon proficiency impacted conversion rates, or to determine individual surgeon volume. Finally, conversion rates may be tied to facility-specific variables that cannot be extracted from the source data. For example, we are unable to determine how long robotic programs have been in place, and if rates of conversion decrease over time. Additionally, we cannot determine reasons for conversion or complications after conversion.
Conclusions
Performing surgery on a robotic platform requires development of a unique skillset, especially for complex robotic surgical procedures. In stratifying facilities by robotic lung cancer resection surgical volume and RAMIE volume, and assessing rates of conversion to open procedures, we found that centers with a low surgical volume for robotic lung cancer resection had higher RAMIE conversion rates than facilities with high robotic lung cancer resection surgical volumes. This finding implies that operative experience in robotics may be transferable between procedures and warrants additional study.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://vats.amegroups.com/article/view/10.21037/vats-23-47/rc
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-23-47/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-23-47/coif). N.R.E. III has received research funding from the Bristol Myers Squibb Foundation, paid to his institution. Additionally, N.R.E. III has received speaker and consultant honoraria from Intuitive, Merck, Bristol Myers Squibb, and Astrazeneca. O.T.O. has received research funding from the Bristol Myers Squibb Foundation, paid to his institution. Additionally, O.T.O. has received speaker honoraria from Intuitive Surgical. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Servais EL, Blasberg JD, Brown LM, et al. The Society of Thoracic Surgeons General Thoracic Surgery Database: 2022 Update on Outcomes and Research. Ann Thorac Surg 2023;115:43-9. [Crossref] [PubMed]
- Rajaram R, Mohanty S, Bentrem DJ, et al. Nationwide Assessment of Robotic Lobectomy for Non-Small Cell Lung Cancer. Ann Thorac Surg 2017;103:1092-100. [Crossref] [PubMed]
- O'Sullivan KE, Kreaden US, Hebert AE, et al. A systematic review and meta-analysis of robotic versus open and video-assisted thoracoscopic surgery approaches for lobectomy. Interact Cardiovasc Thorac Surg 2019;28:526-34. [Crossref] [PubMed]
- Young A, Alvarez Gallesio JM, et al. Outcomes of robotic esophagectomy. J Thorac Dis 2021;13:6163-8. [Crossref] [PubMed]
- Silva JP, Putnam LR, Wu J, et al. Lower Rates of Unplanned Conversion to Open in Robotic Approach to Esophagectomy for Cancer. Am Surg 2023;89:2583-94. [Crossref] [PubMed]
- Manigrasso M, Vertaldi S, Marello A, et al. Robotic Esophagectomy. A Systematic Review with Meta-Analysis of Clinical Outcomes. J Pers Med 2021;11:640. [Crossref] [PubMed]
- Okusanya OT, Sarkaria IS, Hess NR, et al. Robotic assisted minimally invasive esophagectomy (RAMIE): the University of Pittsburgh Medical Center initial experience. Ann Cardiothorac Surg 2017;6:179-85. [Crossref] [PubMed]
- Till BM, Grenda TR, Okusanya OT, et al. Robotic Minimally Invasive Esophagectomy. Thorac Surg Clin 2023;33:81-8. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP, Grosser R, et al. Attaining Proficiency in Robotic-Assisted Minimally Invasive Esophagectomy While Maximizing Safety During Procedure Development. Innovations (Phila) 2016;11:268-73. [Crossref] [PubMed]
- Servais EL, Miller DL, Thibault D, et al. Conversion to Thoracotomy During Thoracoscopic vs Robotic Lobectomy: Predictors and Outcomes. Ann Thorac Surg 2022;114:409-17. [Crossref] [PubMed]
- Halpern AL, Friedman C, Torphy RJ, et al. Conversion to open surgery during minimally invasive esophagectomy portends worse short-term outcomes: an analysis of the National Cancer Database. Surg Endosc 2020;34:3470-8. [Crossref] [PubMed]
- Muslim Z, Stroever S, Poulikidis K, et al. Conversion to Thoracotomy in Non-Small Cell Lung Cancer: Risk Factors and Perioperative Outcomes. Innovations (Phila) 2022;17:148-55. [Crossref] [PubMed]
- Gergen AK, Halpern AL, Helmkamp L, et al. Outcomes After Converted Minimally Invasive to Open Esophagectomy in Patients With Esophageal Cancer. Ann Thorac Surg 2021;112:1593-9. [Crossref] [PubMed]
- Hue JJ, Bachman KC, Worrell SG, et al. Outcomes of robotic esophagectomies for esophageal cancer by hospital volume: an analysis of the national cancer database. Surg Endosc 2021;35:3802-10. [Crossref] [PubMed]
- Drawbert HE, Hey MT, Tarrazzi F, et al. Early discharge on postoperative day 1 following lobectomy for stage I non-small-cell lung cancer is safe in high-volume surgical centres: a national cancer database analysis. Eur J Cardiothorac Surg 2022;61:1022-9. [Crossref] [PubMed]
- Bongiolatti S, Gonfiotti A, Viggiano D, et al. Risk factors and impact of conversion from VATS to open lobectomy: analysis from a national database. Surg Endosc 2019;33:3953-62. [Crossref] [PubMed]
- Reames BN, Ghaferi AA, Birkmeyer JD, et al. Hospital volume and operative mortality in the modern era. Ann Surg 2014;260:244-51. [Crossref] [PubMed]
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128-37. [Crossref] [PubMed]
- Fuchs HF, Harnsberger CR, Broderick RC, et al. Mortality after esophagectomy is heavily impacted by center volume: retrospective analysis of the Nationwide Inpatient Sample. Surg Endosc 2017;31:2491-7. [Crossref] [PubMed]
- Okusanya OT, Hess NR, Luketich JD, et al. Technique of robotic assisted minimally invasive esophagectomy (RAMIE). J Vis Surg 2017;3:116. [Crossref] [PubMed]
- Gandjian M, Williamson C, Sanaiha Y, et al. Continued Relevance of Minimum Volume Standards for Elective Esophagectomy: A National Perspective. Ann Thorac Surg 2022;114:426-33. [Crossref] [PubMed]
- Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-Assisted, Video-Assisted Thoracoscopic and Open Lobectomy: Propensity-Matched Analysis of Recent Premier Data. Ann Thorac Surg 2017;104:1733-40. [Crossref] [PubMed]
- Chen D, Kang P, Tao S, et al. Risk factors of conversion in robotic- and video-assisted pulmonary surgery for non-small cell lung cancer. Updates Surg 2021;73:1549-58. [Crossref] [PubMed]
- Palazzo F, Rosato EL, Chaudhary A, et al. Minimally invasive esophagectomy provides significant survival advantage compared with open or hybrid esophagectomy for patients with cancers of the esophagus and gastroesophageal junction. J Am Coll Surg 2015;220:672-9. [Crossref] [PubMed]
Cite this article as: Collins ML, Whitehorn GL, Mack SJ, Till BM, Rshaidat H, Grenda TR, Evans NR 3rd, Chojnacki KA, Okusanya OT. The association between robotic lung cancer resection and esophagectomy outcomes: a facility-level analysis. Video-assist Thorac Surg 2024;9:3.


