Uniportal video-assisted thoracic surgery lymph node dissection: surgical technique
Highlight box
Surgical highlights
• Uniportal video-assisted thoracic surgery (UVATS) is a valuable technique for the management of non-small cell lung cancer, but concern remains on radicality of mediastinal lymph node dissection. Here, we describe the standardization of our approach for left and right sided systematic mediastinal lymph node dissection performed during UVATS anatomical pulmonary resections. We focus on the patient’s position, the surgical equipment, the incision placement, and exposure tips for en-bloc resection of different lymph node stations.
What is conventional and what is novel/modified?
• Traditionally, VATS requires several incisions for correct exposure and proper dissection during pulmonary anatomical resection. UVATS minimizes surgical trauma access and is potentially associated with decreased post-operative complications and increased quality of life. However, this challenging technique requires experience and specific instrumentation to avoid or minimize instrument clutter/conflict to gain acceptable retraction and exposure through a single utility incision with parallel and coaxial devices.
What is the implication, and what should change now?
• We have shown that complete lymph node dissection can be performed safely and routinely by UVATS.
Introduction
The surgery for non-small cell lung cancer (NSCLC) includes anatomical lung resection and systematic mediastinal lymph node dissection. The involvement of hilar or mediastinal lymph nodes in patients with NSCLC is an important prognostic factor of survival and determines further treatment. For early-stage NSCLC, video-assisted thoracic surgery (VATS) lobectomy has emerged as an effective approach with survival rates comparable to those of classical open thoracotomy (1-6) and better peri-operative outcomes (5-10). Although concerns remain regarding the adequacy and completeness of lymphadenectomy performed by VATS compared to the open thoracotomy approach (11,12), all mediastinal nodal stations are accessible by VATS. The European Society of Thoracic Surgeons (ESTS) has developed guidelines defining the surgical procedure of intraoperative systematic lymph node dissection in patients undergoing resection for NSCLC (13).
Since 2010, there has been the introduction of uniportal VATS (UVATS) as a valid alternative to multiport VATS for pulmonary anatomical resections. We progressively shifted from three-port approach which was started in 2010 to UVATS mastered in 2017. Several technical progresses were important for the transition. Initially, we needed to know how to retract and to expose the pulmonary structures without conflict with instruments through a single utility incision with parallel and coaxial devices. We also endeavor to apply the no-touch technique to avoid tearing the capsule of the lymph nodes. We observed that the use of a high resolution camera with angulation of 30 and dedicated thoracoscopic instruments could achieve acceptable improvements of the surgical technique.
For systematic lymph node dissection, all the lymph nodes with mediastinal fatty tissue are dissected and removed systematically within anatomical landmarks. For right-sided tumours, it is recommended to remove the upper mediastinal nodes (stations 2R and 4R). Ideally, this should be done as an en-bloc resection of all the nodes and fatty tissue within the space limited by the brachiocephalic trunk cranially, the ascending aorta medially, the superior vena cava anteriorly, the esophagus posteriorly and the pulmonary artery inferiorly. To complete right-sided mediastinal lymph node dissection, the lower mediastinum is dissected from the diaphragm to the subcarinal area, including stations 7 (subcarinal), 8 (paraoesophageal) and 9 (pulmonary ligament), must be removed. On the left side, systematic mediastinal lymph node dissection includes resection of stations 5 (aortopulmonary window), 6 (paraaortic), 7 (subcarinal), 8 (paraeophageal) and 9 (pulmonary ligament).
This present article describes the important step for left and right systematic mediastinal lymph node dissection during UVATS anatomical pulmonary resection according to the ESTS guidelines. We focus on patient position, surgical equipment, incision placement and exposure tips for resection of mediastinal lymph node stations. We present this article in accordance with the SUPER reporting checklist (available at https://vats.amegroups.com/article/view/10.21037/vats-23-62/rc).
Preoperative preparation and requirements
The technique of UVATS lymph node dissection is not different from classical open thoracotomy, although the instrumentation and the exposure differ. Patients are discussed at the interdisciplinary tumour board. They are scheduled for a UVATS anatomical pulmonary resection (lobectomy or segmentectomy) and systematic mediastinal lymph node dissection if they present a peripheral cT1 or cT2 tumour not visible by bronchoscopy, and if no hilar or mediastinal lymph node involvement is suspected on positron emission tomography (PET) scan. Mediastinoscopy is not routinely performed before procedure. We traditionally perform lymph node dissection after completion of anatomical resection although some surgeons prefer to first rule out nodal involvement with frozen section in case of neo-adjuvant treatment to assess the resecability or in case of suspicious lymph node involvement when a pulmonary segmentectomy is planned.
The general anesthesia and ventilation are managed with a double-lumen tube. The patient is positioned on the side and the intercostal distance is increased by flexing the table at the tip of the scapula. We perform a 4-cm incision in the 4th or 5th intercostal space (depending if it is an upper and lower lobectomies, respectively) between the tip of the scapula and the breast, on the anterior axillary line. We use a skin retractor (Alexis® Retractor Applied Medical, California, USA), to protect the wound. The surgeon and assistant are positioned in the front of the patient, whereas the scrub stand posterior to the patient.
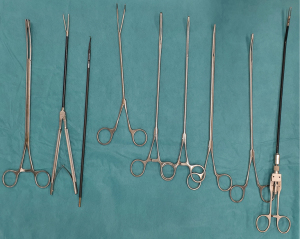
For UVATS procedures, we recommend the use of a 10-mm HD with 30° angulation camera which allows complete visualization of all pleural cavity. Specific instruments are used with both proximal and distal articulation avoiding interference with intercostal space: dissector, node grasper, Duval grasper, Foester clamp, Debakey forceps (Scanlan®, Minnesota, USA) (Figure 1). The monopolar hook cautery is routinely used for the lymph node dissection allowing precise dissection, however some caution is necessary since electrocautery lesions may appear in unexperienced hands during dissection. Alternatively, ultrasonic shears or a bipolar sealing instrument may be useful for hemostatic purposes, like Ultracision Harmonic scalpel (Ethicon, Johnson & Johnson, New Jersey, USA), or Ligasure (Medtronic, Minnesota, USA). Additional hemostasis may be obtained using a Tabotamp™ fibrillar fleece from Ethicon©. A suction device is mandatory during dissection maneuver for immediate evacuation of smoke and exposure. A sponge stick with open thoracotomy instruments is available in the operating room for immediate control of hemorrhage.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this study, accompanying images and videos. A copy of the written consent is available for review by the editorial office of this journal.
Step-by-step description
Left side
The order of the left-sided systematic mediastinal lymph node dissection is as follows: stations 9 and 8, station 7 and stations 5 and 6 (Video 1).
Stations 9 and 8
The lung is retracted superiorly exposing the inferior pulmonary ligament which is divided with the Ligasure device or with the hook until the inferior pulmonary vein is identified. Stations 9 and 8 become visible and the dissection is performed close to the lymph nodes to avoid injury to the esophagus, the vagus nerve and the inferior pulmonary vein.
Station 7
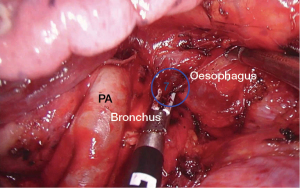
The lung is retracted anteriorly. The dissection of station 7 from the left side may be difficult since the nodes are deeply located between the esophagus and the two main bronchi and the aorta. We usually use a large sponge introduced by the utility incision for exposure. The camera is inserted posteriorly and a sponge or lung grasper is used to retract the lung anteriorly. Care and meticulous retraction are mandatory to avoid vascular or bronchial stump lesions, as well as damage to the esophagus. The pleura is opened on the posterior hilum along the inferior border of the left lower lobe bronchus and left main bronchus. The carina is progressively cleared and the use of bipolar instrument greatly facilitates the dissection along the main bronchus and the esophagus (Figure 2). Injury of the bronchial artery can appear in case of excessive traction on lymph nodes whose control can be difficult. The left side subcarinal nodal dissection is significantly easier after a left lower lobectomy thanks to the division of the inferior pulmonary vein. The use of a right double lumen tube improves significantly the visibility and exposure of this area by avoiding the rigidity of the main left bronchus in case of left double lumen tube. Sometimes, increasing the tidal volume of the right lung may improve the exposure of the subcarinal space.
Stations 5 and 6
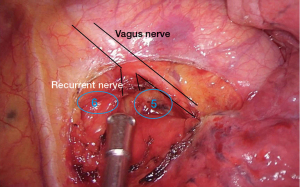
The lung is retracted inferiorly and posteriorly. The mediastinal pleura is opened in front of the aortopulmonary window and the phrenic and vagus nerves should be visualized and preserved. Pleural flaps are gently moved away, and the left recurrent laryngeal nerve is identified. Finally, we removed all lymph nodes located between the vagus and phrenic nerves which corresponds to the station 6 and all those located adjacent and posteriorly to the vagus nerve corresponding to station 5 (Figure 3).
Right side
Right-sided mediastinal lymph node dissection is generally performed as follows: stations 9 and 8, station 7 and stations 4R and 2R (Video 2).
Stations 9 and 8
The right lower lobe is pushed superiorly with a grasper or with a sponge that is introduced through the utility incision. The inferior pulmonary ligament is then progressively divided to identify the inferior pulmonary vein using the hook or the bipolar device introduced by the same incision. Stations 9 and 8 can be easily seen and removed gently with blunt dissection.
Station 7
The station 7 is easier to access on the right side than on the left side because the vision of the subcarinal area is more direct on the right side. However, a sponge or lung grasper are generally required to retract the lung anteriorly. The camera is introduced posteriorly. First, the posterior mediastinal pleura is incised in front of the esophagus up to the azygos arch with the hook. Small esophageal arteries can be cut by using electrocautery or bipolar instruments. Then, the incision joins the visceral pleura covering the bronchial system. The next step is to dissect the border of the bronchus intermedius in a retrograde fashion, ultimately leading to the subcarinal lymph node station. A combination of sharp and blunt dissection is used to reach station 7 and to remove the lymph nodes (Figure 4). The nodes area includes the pericardium anteriorly, the esophagus posteriorly, and the carina superiorly. The bronchial artery usually found in this position is generally cauterized with the Ligasure device or can be controlled by the application of a clip. After removal of the lymph nodes, the right and left mainstem bronchi are clearly visualized since all fatty tissue has been removed. Occasionally, a Tabotamp fibrillar fleece is placed in the subcarinal region to improve hemostasis.
Stations 4R and 2R
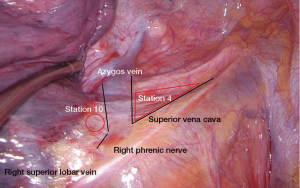
First—if there was no prior upper lobectomy—we pull the right upper lobe inferiorly and posteriorly. The mediastinal pleura is incised on the inferior aspect of the azygos vein. We start with the dissection of the 10R lymph nodes, which are close to the pulmonary artery and below the azygos vein. Then, we continue with the dissection of 4R nodes. Transection of the azygos vein may be required in selected cases, particularly after induction therapy. With a combination of blunt and sharp dissection, we perform an en-bloc excision of all the lymphatic and fatty tissue between the superior vena cava, the pericardium covering ascending aorta and the trachea from the level of the pulmonary artery to the innominate artery. This removes all lymph nodes from stations 4R and 2R. The vagus nerve is identified and preserved. Often, a small vein arising from the superior vena cava drains the level 4R nodes. It should be identified and clipped. Finally, a Tabotamp fibrillar fleece can be placed in the bed of the dissected lymph node stations to ensure hemostasis.
Postoperative considerations
At the end of the procedure, we usually realize an intercostal nerve bloc with local anesthesia between the 2nd and 8th intercostal space. A 24 French chest tube is inserted through the utility incision, and the lung is re-ventilated under direct vision. An enhanced recovery after surgery (ERAS) pathway is routinely post-operatively applied for all patients.
Discussion
According to the ESTS guidelines regarding management of NSCLC, systematic mediastinal lymph node dissection is an imperative part of the surgery for accurate staging and prognostic purposes (13,14). Indeed, despite improvements of preoperative mediastinal staging by positron emission tomography (PET) imaging, about 10% to 20% of PET-negative patients will show pathological mediastinal nodal involvement even in cT1 tumors (13). Thus, intraoperative lymph node dissection is necessary to allow a precise staging for adjuvant therapies adaptation and optimization. We traditionally perform a systematic lymph node dissection including at least three mediastinal nodal stations as recommended by the ESTS guidelines. Additionally, the hilar and the intrapulmonary lymph nodes are dissected as well. All the mediastinal tissue containing the lymph nodes is dissected and removed systematically within anatomical landmarks: on the right side we remove stations 2R, 4R, 10R, 7, 8R and 9R. On the left side, the stations 5, 6, 10L, 7, 8L, 9L are removed. The stations 4L is rarely routinely removed for early stage lung cancer since the exposure requires generally the division of the ligamentum arteriosum with risk of laryngeal nerve palsy.
Since the development of minimally invasive VATS for the treatment of NSCLC, several studies demonstrated equivalent efficacy of lymphadenectomy performed by VATS or open thoracotomy approach (11,15). UVATS has been developed with the idea of further reducing surgical trauma. It demonstrated favorable results regarding postoperative outcomes in several meta-analyses and propensity-matched studies as compared to multiportal VATS (16-19).
Despite the non-negligible learning curve to master anatomical pulmonary resections with lymph node dissection by UVATS, this approach is not inferior to open thoracotomy or multiportal VATS in terms of oncological results, operative time or bleeding. Indeed, Ismail et al. demonstrated in their retrospective study including 136 patients undergoing UVATS anatomical pulmonary resections for NSCLC that the mean number of resected lymph nodes (20.14±10.73) was similar to the number reported in the literature for thoracotomy, VATS and robotic-assisted thoracic surgery (RATS) approaches (20). In the same way, another series including 453 patients with NSCLC undergoing anatomical pulmonary resections (197 UVATS, 256 multiportal VATS) demonstrated a similar number and stations of lymph nodes dissected as well as similar 1 – (77.65% vs. 79.3%; P=0.517), 2 – (68.53% vs. 66.02%; P=0.543) and 3-year (58.38% vs. 55.47%; P=0.106) survival rates between groups (19). Moreover, UVATS patients demonstrated better postoperative outcomes with reduced blood loss, shorter length of drainage and postoperative length of stay (19). Nachira et al. corroborated these results with similar mean number of resected lymph nodes in their propensity-matched patients, whether for the thoracotomy (15.50±7.84) or UVATS (16.53±8.66) approach (P=0.785). There was also no difference in the 3-year overall survival (74% vs. 78%; P=0.204) and 3-year disease-free survival (66% vs. 62%; P=0.917) rates between their groups (21). Pathological nodal upstaging was also similar between groups (23.9% vs. 17.4%; P=0.44) and regression analysis showed that the type of surgical approach was not a significant risk factor for nodal upstaging (P=0.445) (21).
UVATS may potentially increase the risk of complications associated with worse exposure or procedure learning curve. Possible complications include bleeding; laryngeal or phrenic nerve palsy and chylothorax. We have always a sponge ready for compression in case of bleeding. The use of bipolar cautery is very useful for dissection, particularly to control the bronchial arteries on station 7. Phrenic and laryngeal nerves should be clearly identified on stations 5 and 6 before coagulation is used. Sometimes the use of small peanut for blunt dissection near nerves may help to identify them and avoid thermic damage.
Conclusions
In conclusion, systematic mediastinal lymphadenectomy by UVATS is feasible and safe for stations 2R, 4R, 7, 8, 9 on the right side and stations 5, 6, 7, 8, 9 on the left side. UVATS allows an optimal visualization and proper dissection of mediastinal lymph nodes, according to guidelines established for the treatment of NSCLC.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Carlos Galvez Munoz and Paula A. Ugalde Figueroa) for the series “Advanced Uniportal VATS” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://vats.amegroups.com/article/view/10.21037/vats-23-62/rc
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-23-62/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-23-62/coif). The series “Advanced Uniportal VATS” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this study, accompanying images and videos. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [Crossref] [PubMed]
- Walker WS, Codispoti M, Soon SY, et al. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg 2003;23:397-402. [Crossref] [PubMed]
- Roviaro G, Varoli F, Vergani C, et al. Long-term survival after videothoracoscopic lobectomy for stage I lung cancer. Chest 2004;126:725-32. [Crossref] [PubMed]
- Kim K, Kim HK, Park JS, et al. Video-assisted thoracic surgery lobectomy: single institutional experience with 704 cases. Ann Thorac Surg 2010;89:S2118-22. [Crossref] [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8. [Crossref] [PubMed]
- Cheng D, Downey RJ, Kernstine K, et al. Video-assisted thoracic surgery in lung cancer resection: a meta-analysis and systematic review of controlled trials. Innovations (Phila) 2007;2:261-92. [Crossref] [PubMed]
- Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;83:1965-70. [Crossref] [PubMed]
- Yim AP, Wan S, Lee TW, et al. VATS lobectomy reduces cytokine responses compared with conventional surgery. Ann Thorac Surg 2000;70:243-7. [Crossref] [PubMed]
- Whitson BA, D'Cunha J, Andrade RS, et al. Thoracoscopic versus thoracotomy approaches to lobectomy: differential impairment of cellular immunity. Ann Thorac Surg 2008;86:1735-44. [Crossref] [PubMed]
- Petersen RP, Pham D, Burfeind WR, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg 2007;83:1245-9; discussion 1250. [Crossref] [PubMed]
- Watanabe A, Koyanagi T, Ohsawa H, et al. Systematic node dissection by VATS is not inferior to that through an open thoracotomy: a comparative clinicopathologic retrospective study. Surgery 2005;138:510-7. [Crossref] [PubMed]
- Denlinger CE, Fernandez F, Meyers BF, et al. Lymph node evaluation in video-assisted thoracoscopic lobectomy versus lobectomy by thoracotomy. Ann Thorac Surg 2010;89:1730-5; discussion 1736. [Crossref] [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- D'Amico TA, Niland J, Mamet R, et al. Efficacy of mediastinal lymph node dissection during lobectomy for lung cancer by thoracoscopy and thoracotomy. Ann Thorac Surg 2011;92:226-31; discussion 231-2. [Crossref] [PubMed]
- Xie D, Zhong Y, Deng J, et al. Comparison of uniportal video-assisted thoracoscopic versus thoracotomy bronchial sleeve lobectomy with pulmonary arterioplasty for centrally located non-small-cell lung cancer. Eur J Cardiothorac Surg 2021;59:978-86. [Crossref] [PubMed]
- Xiang Z, Wu B, Zhang X, et al. Uniportal versus multiportal video-assisted thoracoscopic segmentectomy for non-small cell lung cancer: a systematic review and meta-analysis. Surg Today 2023;53:293-305. [Crossref] [PubMed]
- Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg 2016;5:76-84. [Crossref] [PubMed]
- Wu HR, Liu CQ, Xu MQ, et al. Systematic mediastinal lymph node dissection outcomes and conversion rates of uniportal video-assisted thoracoscopic lobectomy for lung cancer. ANZ J Surg 2019;89:1056-60. [Crossref] [PubMed]
- Ismail M, Nachira D, Swierzy M, et al. Lymph node upstaging for non-small cell lung cancer after uniportal video-assisted thoracoscopy. J Thorac Dis 2018;10:S3648-54. [Crossref] [PubMed]
- Nachira D, Congedo MT, Tabacco D, et al. Surgical Effectiveness of Uniportal-VATS Lobectomy Compared to Open Surgery in Early-Stage Lung Cancer. Front Surg 2022;9:840070. [Crossref] [PubMed]
Cite this article as: Forster C, Gonzalez M. Uniportal video-assisted thoracic surgery lymph node dissection: surgical technique. Video-assist Thorac Surg 2024;9:10.