Pancoast tumors
Introduction
Tumors originating from the superior pleuro-pulmonary margin above the first rib that impact adjacent thoracic inlet structures can lead to a clinical presentation termed Pancoast syndrome (1). This condition manifests with pain dispersing to areas like the neck, axilla, anterior thoracic wall, the inner side of the scapula, and the same side’s inner arm and forearm extending to the wrist. It may also show intrinsic hand muscle weakness and atrophy, Claude Bernard Horner manifestation, and swelling of the upper arm (2). These manifestations arise from the involvement of multiple structures: the parietal pleura, endothoracic fascia, apical bone structure, and nerves including the brachial plexus, sympathetic chain, and stellate ganglion (3-6). Tumors located in the superior sulcus region possess the potential to and notably infiltrate the subclavian vessels. The involvement of subclavian vessels can have significant clinical implications due to their pivotal role in blood flow to the upper extremity. Furthermore, the inferior segment of the brachial plexus and the superior terminus of the thoracic autonomic chain, encompassing the stellate ganglion, can also be affected. The manifestation of neurologic indications and symptoms stemming from the invasion of the brachial plexus is recognized as Pancoast syndrome. Concurrently, tumoral destruction of the stellate ganglion culminates in the clinical presentation termed Horner syndrome (4-6).
Most Pancoast tumors are non-small cell pulmonary carcinomas, making up 3% to 5% of pulmonary carcinoma instances. The predominant histological variant is adenocarcinoma, transitioning from the earlier predominant squamous cell carcinoma (7). This carcinoma variant is predominantly observed in industrialized nations and could be associated with filtered cigarette consumption. Pancoast syndrome can also result from other cancers and even non-malignant tumors in the superior pulmonary sulcus (8,9). On rare occasions, other tumors like paravertebral thoracic schwannomas and myxofibrosarcomas can lead to analogous clinical observations (10-12).
The aim of this article is to delineate the key diagnostic and therapeutic considerations for patients presenting with Pancoast syndrome, highlighting the importance of comprehensive evaluation, staging, and the role of the video-assisted thoracic surgery (VATS) approach in management.
Evaluation
In the context of diagnosing Pancoast tumors, it’s crucial to evaluate various imaging tools and diagnostic methodologies at our disposal. Simple thoracic radiography might not identify the tumor during its initial phase. As the pathology advances, one might observe lung apex disparities or enhanced pleural thickness. Computed tomography (CT) can reveal encroachments into bones, spine, mediastinum, or the brachial plexus. Conversely, magnetic resonance imaging (MRI) offers a detailed evaluation of the tumor’s local progression (13-16).
Over the recent decade, the utilization of positron emission tomography (PET) has seen a marked escalation as an integral component of the preoperative evaluation for lung cancer. Within the context of Pancoast tumors, its relevance manifests in dual capacities. Primarily, it facilitates the preoperative staging of lymph nodes (17) and aids in the detection of occult metastases in patients with non-small cell lung cancer (NSCLC) (2). Secondly, PET is instrumental in re-stratifying tumors subsequent to neoadjuvant therapy (18). However, its limitations include an inability to offer precise topographical details regarding the initial lesion, except in the presence of coincident atelectasis. Lymph nodes that display positivity on a PET scan necessitate verification via mediastinoscopy or endoscopic endobronchial ultrasound (EBUS). Moreover, a negative PET-CT scan does not conclusively negate nodal engagement, hence, invasive staging remains indispensable. For cases presenting with intrinsic hand muscle atrophy, a rigorous neurological assessment is mandated to discern nerve root compromise.
Utilizing a flexible fiberoptic bronchoscope for bronchoscopy may help identify any invasion into tracheal or bronchial spaces, but acquiring a tissue sample is crucial for histological validation, gauging surgical feasibility, and strategizing treatment (19).
When seeking tissue diagnosis, percutaneous transthoracic needle biopsy emerges as the premier method, boasting a diagnostic efficacy of 95% (19). In situations where the common and less invasive methods render ambiguous results, VATS might be employed for tissue sampling. Additionally, employing EBUS, mediastinoscopy—particularly on the right side—and/or anterior mediastinotomy could be pivotal in delineating the nodal status and disease’s spread. EBUS allows access to most mediastinal, hilar, interlobar, and selected intralobar lymph nodes because the lymph nodes are anatomically closely associated with the airways. This explains why EBUS-transbronchial needle aspiration (TBNA) was more accurate than mediastinoscopy for staging NSCLC in certain studies (20).
It’s imperative to procure histological evidence when there’s an apparent enlargement of mediastinal lymph nodes from radiographic or CT evaluations (21-23).
It’s worth emphasizing that while certain diagnostic tools may exhibit exceptional sensitivity and specificity, their collective use ensures a thorough understanding of the pathology.
The initiation of preoperative physiological evaluation encompasses a comprehensive cardiovascular examination followed by spirometric assessment to ascertain the forced expiratory volume in 1 second (FEV1) and the diffusing capacity of the lung for carbon monoxide (DLCO). It is imperative to compute the anticipated postoperative pulmonary functions (PPO). When both % PPO FEV1 and % PPO DLCO exceed 60%, the individual is deemed to be at negligible risk for anatomical lung resection, obviating the need for supplementary assessments (23).
Should the % PPO FEV1 or % PPO DLCO fall within the range of 60% to 30% of the projected values, it becomes requisite to employ a low-technology exercise assessment for more intricate evaluation. Demonstrable proficiency on this rudimentary exercise assessment, evidenced by ascending stairs beyond 22 m or achieving a shuttle walk distance exceeding 400 m, categorizes the risk associated with anatomical resection as insubstantial (23).
In instances where PPO FEV1 or PPO DLCO (or both) are below the 30% threshold or if results from the stair-climbing or shuttle walk test are deemed unsatisfactory, a cardiopulmonary exercise test becomes paramount. A peak oxygen consumption (O2peak) falling below 10 mL/kg/min or 35% signifies a pronounced risk of mortality and prolonged debilitation subsequent to major anatomical resection. Conversely, an anticipated O2peak surpassing 20 mL/kg/min or 75% is indicative of minimal risk (21,23). In addition, the patient’s performance status and renal and neurologic function must be adequate for platinum-containing chemotherapy (21).
Staging
The classification of Pancoast tumors, based on their T status, is determined as a minimum of T3 due to the invasion into the chest wall. Should there be further intrusion into the vertebral structure or subclavian vessels, it escalates to T4. Research data indicates a bleak 5-year survival prognosis, less than 10%, for patients with vertebral infiltration, while subclavian vessel compromise also indicates poor outcomes (24,25). A study by Dartevelle and colleagues documented a 30% 5-year survival for T4 cases, however, they highlighted the detrimental effect of subclavian vessel compromise (22).
Preoperative identification of pN2 or pN3 node engagement is paramount, given their association with suboptimal 5-year survival outcomes. Pancoast tumors present with mediastinal N2 lymph node positivity in roughly 20% of cases, underscoring the pivotal role of mediastinoscopy for preoperative assessment as asserted by earlier research (22). The integration of chemoradiotherapy and extensive surgical removal advocates for an intensified extrathoracic assessment, even in cases devoid of distant metastatic signs (26).
In an expansive evaluation of superior sulcus tumors conducted at Memorial Sloan-Kettering Cancer Center, the cohort subjected to bimodal intervention (initial radiotherapy succeeded by comprehensive removal) manifested a 5-year survival of 46% for stage IIB, none for stage IIIA, and 13% for stage IIIB malignancies. Factors like T and N classifications and the thoroughness of tumor removal influenced survival rates. Comprehensive staging played a cardinal role in survival outcomes. Yet, complete histological tumor removal was realized in just 64% of T3 N0 and a mere 39% of T4 N0 malignancies (27).
Rusch et al. [2000] have undertaken pivotal research to understand the factors that influence the outcomes post-surgical resection of T3 and T4 lung cancers of the superior sulcus. Their comprehensive study reveals pertinent findings that add depth to our understanding of the surgical management of such cancers (24).
In a subsequent study conducted by Rusch et al. in 2007, an analysis of superior sulcus NSCLCs was carried out. The results shed light on the effects of induction chemoradiation followed by surgical resection on the long-term outcomes of these patients (28).
Furthermore, the Intergroup Trial 0160 (SWOG 9416) from 2001 focused on evaluating patients at stage T3–4N0–1. These patients underwent two cycles of cisplatin-etoposide in conjunction with a concurrent radiotherapy dosage of 45 Gy. The results were promising: complete resections were observed in 91% of T3 patients and 87% of T4 patients. The post-surgical mortality rate was an impressive low of 2.3%. Upon a detailed pathological examination, a third of the cases exhibited complete remissions, while another third showed microscopic residual disease. The remaining third presented with macroscopic viable tumor. Importantly, the pathologic complete response emerged as the primary prognostic determinant for survival (29).
Treatment
Surgical intervention for Pancoast tumors is intricate, attributed to the tumor’s encroachment into nearby structures like the brachial plexus, subclavian vessels, and vertebral column. Multiple surgical protocols exist, with the prevalent method being the comprehensive removal of the tumor alongside the chest wall, generally undertaken 3 to 5 weeks post-chemo-radiotherapy (post-CT/RT). The extent of removal is influenced by the tumor’s dimensions and positioning, with the posterior and anterior trans-cervical strategies being frequently employed.
Primary method involves complete removal of the tumor and part of the chest wall, typically 3–5 weeks after CT/RT.
Procedure type is dictated by the tumor’s size and location. Common methods include the posterior and anterior trans-cervical approaches.
If the tumor affects the brachial plexus or vertebral column, a combined thoracic and neurosurgical approach is required.
Advancements in spinal equipment now allow for improved removal from the vertebral structure.
Certain structures’ involvements may pose surgical challenges, but not always contraindications.
Engagements of specific structures like the vertebral elements, subclavian conduit, and metastasis to same-sided supraclavicular lymph nodes might be deemed as potential, though not definite, surgical restrictions by certain experts (17,22,30-34).
Post-surgery complications can include chylothorax, ulnar nerve issues, Horner’s syndrome, cerebrospinal fluid-related problems such as leaks and meningitis.
Typically, the removal of T1 and T2 neural roots doesn’t cause significant clinical issues. Reported surgical mortality rates range from 4% to 10% (35).
For cases where the tumor is regionally progressive, surgically unfeasible, or if surgery poses medical risks, CT/RT or sole radiotherapy stands as a feasible therapeutic alternative.
Synchronous CT/RT is recommended for stage III non-Pancoast NSCLC.
Follow-up immunotherapy is advised for a year after definitive CT/RT.
Primary radiotherapy is suitable for patients with deteriorated health or metastatic tumors. It can provide pain relief for approximately 90% of patients (36-38).
Intense radiotherapy can lead to 5-year survival rates up to 40% for localized superior sulcus tumors. Dosages between 60 and 66 Gy for infeasible tumors and reduced dosages for symptom relief in metastatic patients (36-38).
Traditionally, sole radiation therapy (RT) before tumor resection resulted in higher incomplete resections, increased local recurrence, and suboptimal survival rates.
Induction CT/RT has shown to significantly reduce both local and distant recurrences and improve survival rates for stage III lung cancer.
The strategy of induction CT/RT followed by resection is more effective than relying solely on induction RT (30).
For a comprehensive treatment guideline for patients with bronchogenic carcinoma, including a detailed treatment scheme for Pancoast tumors, readers are referred to the National Comprehensive Cancer Network guidelines (39).
Uniportal VATS for Pancoast tumor
Various methodologies are adopted for VATS, including the standard VATS lobectomy, necessitating access incisions measuring between 1.5 and 2 cm for 2 to 4 thoracoscopic portals and 2–6 cm anterior utility incision. The portal count relies on the surgeon’s proficiency. This modality is minimally invasive as rib spreading in intercostal spaces is not mandated. Literature differentiates between conventional VATS (c-VATS) and advanced VATS (a-VATS), with the initial technique operating under camera supervision and the latter through video-assisted mini-thoracotomy (40). Technological strides have fostered the evolution of these methodologies, escalating the ubiquity of VATS lobectomy clinically. STS data indicates a rise in VATS lobectomy preference from 10% in 2002 to 29% in 2007 compared to traditional surgical techniques (41).
In the VIOLET study undertaken by the academic department of thoracic surgery at the Royal Brompton Hospital, London, in 2022 (42), it was elucidated that patients undergoing VATS lobectomy for lung cancer exhibited a superior recovery in physical function within the 5-week post-randomization period in comparison to those subjected to open surgery.
In Pancoast procedures, which entail expansive incisions, VATS diminishes pain and complications (43). The minimized invasiveness from VATS facilitates patients in undergoing robust physical therapy and enhanced mobility (41-43). For Pancoast tumor excisions, VATS must be performed only when maintaining the oncological principles as in open surgery. Nonetheless, its suboptimal application may yield inferior outcomes, and it should be reserved for adept thoracic surgeons. Conventional thoracotomy remains advisable when a surgeon’s VATS experience is minimal or in the face of perilous perioperative challenges. Complication rates post-VATS lobectomy range from 6% to 34.2% as opposed to a ceiling of 58% in standard thoracotomy (44-49).
Uniportal VATS has emerged as a novel, proven, and feasible strategy since the early 1990s, with the latest progression being its uniportal form. The VATS technique for lobectomy isn’t rigid, typically comprising 3 to 4 incisions but also achievable through a solitary incision. This uniportal strategy was pioneered by Rocco and team for select thoracic procedures (46). The creation of flexible instruments has paved the way for major pulmonary excisions via a singular incision. Rocco and associates first introduced single-incision pulmonary excisions in 2004. By 2011, Gonzalez and his team from Coruña University Hospital presented the initial uniportal VATS lobectomy (47). The incision magnitude aligns with that typically reserved for dual or triple portal access (50-53). Instrumentation is facilitated by high-caliber 30° thoracoscopes, offering an unobstructed target view. For Pancoast tumors, a hybrid strategy is employed, which merges uniportal pulmonary removal with direct vision chest wall excision (53).
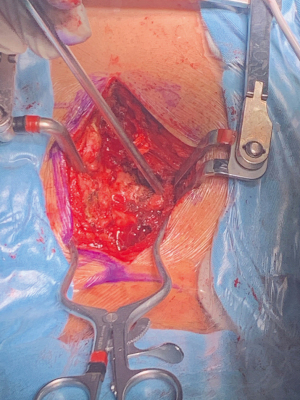
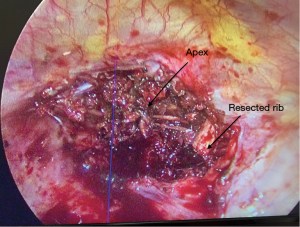
In instances of vertebral involvement, surgical initiation involves positioning the patient prone (Figure 1). Following the tumor’s disengagement from the spine and potential mesh placement for spinal cord protection, the tethered pulmonary lobe is disengaged and relocated into the pleural cavity. Subsequently, the patient is repositioned into the decubitus stance. The lobectomy proceeds as customary with the uniportal modality. Post-surgery, the excised region undergoes internal exploration (Figure 2). Occasionally, posterior incision digital palpation aids in tumor localization, ensuring no residual suspect tissues (Video 1). Concluding the operation, the chest drain is positioned through the same incision, with layered wound closure (Figure 3).
Strengths and limitations
Strengths
The article provides an analysis of VATS in treating Pancoast tumors, offering valuable insights into its history, variations, and technological advancements.
It presents a comprehensive view of the clinical implications, including patient recovery and pain mitigation, and discusses the significance of VATS in thoracic oncology.
The exploration of the uniportal VATS and hybrid approaches contributes to understanding the range of options available for complex surgical scenarios.
Limitations
The article may not sufficiently address the specific challenges and potential complications associated with each technique, particularly in varied clinical settings. While it emphasizes the need for surgical expertise, it may not provide detailed guidance on the learning curve or training requirements for these advanced techniques. The focus on VATS might limit the discussion of alternative treatments or approaches that could be relevant in certain patient scenarios.
Conclusions
Pancoast tumors persist as a formidable surgical challenge. Leveraging VATS techniques can expedite recovery, curtail post-surgical pain, and minimize complications, contingent on preserving oncological principles and its execution by skilled surgeons.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Carlos Galvez Munoz and Paula A. Ugalde Figueroa) for the series “Advanced Uniportal VATS” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-23-3/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-23-3/coif). The series “Advanced Uniportal VATS” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was not obtained from the patients for the publication of this article and accompanying images as all the images and video were anonymous without showing the patients face identity.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arcasoy SM, Jett JR. Superior pulmonary sulcus tumors and Pancoast's syndrome. N Engl J Med 1997;337:1370-6. [Crossref] [PubMed]
- Kratz JR, Woodard G, Jablons DM. Management of Lung Cancer Invading the Superior Sulcus. Thorac Surg Clin 2017;27:149-57. [Crossref] [PubMed]
- Peedell C, Dunning J, Bapusamy A. Is there a standard of care for the radical management of non-small cell lung cancer involving the apical chest wall (Pancoast tumours)? Clin Oncol (R Coll Radiol) 2010;22:334-46. [Crossref] [PubMed]
- Detterbeck FC. Changes in the treatment of Pancoast tumors. Ann Thorac Surg 2003;75:1990-7. [Crossref] [PubMed]
- Marulli G, Battistella L, Mammana M, et al. Superior sulcus tumors (Pancoast tumors). Ann Transl Med 2016;4:239. [Crossref] [PubMed]
- Bruzzi JF, Komaki R, Walsh GL, et al. Imaging of non-small cell lung cancer of the superior sulcus: part 1: anatomy, clinical manifestations, and management. Radiographics 2008;28:551-60; quiz 620. [Crossref] [PubMed]
- Munir M, Jamil SB, Rehmani S, et al. Pancoast-Tobias Syndrome: A Unique Presentation of Lung Cancer. Cureus 2021;13:e13112. [Crossref] [PubMed]
- Stanford W, Barnes RP, Tucker AR. Influence of staging in superior sulcus (Pancoast) tumors of the lung. Ann Thorac Surg 1980;29:406-9. [Crossref] [PubMed]
- Shahian DM, Neptune WB, Ellis FH Jr. Pancoast tumors: improved survival with preoperative and postoperative radiotherapy. Ann Thorac Surg 1987;43:32-8. [Crossref] [PubMed]
- HERBUT PA. WATSON JS. Tumor of the thoracic inlet producing the Pancoast syndrome; a report of 17 cases and a review of the literature. Arch Pathol (Chic) 1946;42:88-103. [PubMed]
- Goldman SM, Fajardo AA, Naraval RC, et al. Metastatic transitional cell carcinoma from the bladder: radiographic manifestions. AJR Am J Roentgenol 1979;132:419-25. [Crossref] [PubMed]
- Alshammari FA, Alotaibi AM, Alali MA, et al. Schwannoma: A Rare Etiology of Pancoast Syndrome. Cureus 2021;13:e19418. [Crossref] [PubMed]
- Takasugi JE, Rapoport S, Shaw C. Superior sulcus tumors: the role of imaging. J Thorac Imaging 1989;4:41-8. [Crossref] [PubMed]
- Łapiński M, Dziadziuszko R, Sawicka W, et al. Early results of a trimodality treatment for superior sulcus tumors. Kardiochir Torakochirurgia Pol 2014;11:268-72. [Crossref] [PubMed]
- Laissy JP, Soyer P, Sekkal SR, et al. Assessment of vascular involvement with magnetic resonance angiography (MRA) in Pancoast syndrome. Magn Reson Imaging 1995;13:523-30. [Crossref] [PubMed]
- Heelan RT, Demas BE, Caravelli JF, et al. Superior sulcus tumors: CT and MR imaging. Radiology 1989;170:637-41. [Crossref] [PubMed]
- Narayan S, Thomas CR Jr. Multimodality therapy for Pancoast tumor. Nat Clin Pract Oncol 2006;3:484-91. [Crossref] [PubMed]
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:7S-37S.
- Paulson DL, Weed TE, Rian RL. Cervical approach for percutaneous needle biopsy of Pancoast tumors. Ann Thorac Surg 1985;39:586-7. [Crossref] [PubMed]
- Yang PC, Lee LN, Luh KT, et al. Ultrasonography of Pancoast tumor. Chest 1988;94:124-8. [Crossref] [PubMed]
- Burke JR, Duarte IG, Thourani VH, et al. Preoperative risk assessment for marginal patients requiring pulmonary resection. Ann Thorac Surg 2003;76:1767-73. [Crossref] [PubMed]
- Dartevelle PG, Chapelier AR, Macchiarini P, et al. Anterior transcervical-thoracic approach for radical resection of lung tumors invading the thoracic inlet. J Thorac Cardiovasc Surg 1993;105:1025-34. [Crossref] [PubMed]
- Paulson DL. Technical considerations in stage III disease: the" superior sulcus" lesion. In: Delarue NC, Eschapasse H, editors. International trends in general thoracic surgery. Philadelphia: Saunders; 1985:121-33.
- Rusch VW, Parekh KR, Leon L, et al. Factors determining outcome after surgical resection of T3 and T4 lung cancers of the superior sulcus. J Thorac Cardiovasc Surg 2000;119:1147-53. [Crossref] [PubMed]
- Paulson DL. Carcinomas in the superior pulmonary sulcus. J Thorac Cardiovasc Surg 1975;70:1095-104. [Crossref] [PubMed]
- Martinod E, D'Audiffret A, Thomas P, et al. Management of superior sulcus tumors: experience with 139 cases treated by surgical resection. Ann Thorac Surg 2002;73:1534-9; discussion 1539-40. [Crossref] [PubMed]
- Gandhi S, Walsh GL, Komaki R, et al. A multidisciplinary surgical approach to superior sulcus tumors with vertebral invasion. Ann Thorac Surg 1999;68:1778-84; discussion 1784-5. [Crossref] [PubMed]
- Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Clin Oncol 2007;25:313-8. [Crossref] [PubMed]
- Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for non-small cell lung carcinomas of the superior sulcus: Initial results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Thorac Cardiovasc Surg 2001;121:472-83. [Crossref] [PubMed]
- York JE, Walsh GL, Lang FF, et al. Combined chest wall resection with vertebrectomy and spinal reconstruction for the treatment of Pancoast tumors. J Neurosurg 1999;91:74-80. [Crossref] [PubMed]
- Wright CD, Moncure AC, Shepard JA, et al. Superior sulcus lung tumors. Results of combined treatment (irradiation and radical resection). J Thorac Cardiovasc Surg 1987;94:69-74. [Crossref] [PubMed]
- Hagan MP, Choi NC, Mathisen DJ, et al. Superior sulcus lung tumors: impact of local control on survival. J Thorac Cardiovasc Surg 1999;117:1086-94. [Crossref] [PubMed]
- Neal CR, Amdur RJ, Mendenhall WM, et al. Pancoast tumor: radiation therapy alone versus preoperative radiation therapy and surgery. Int J Radiat Oncol Biol Phys 1991;21:651-60. [Crossref] [PubMed]
- Attar S, Krasna MJ, Sonett JR, et al. Superior sulcus (Pancoast) tumor: experience with 105 patients. Ann Thorac Surg 1998;66:193-8. [Crossref] [PubMed]
- Shigemura N, Akashi A, Funaki S, et al. Long-term outcomes after a variety of video-assisted thoracoscopic lobectomy approaches for clinical stage IA lung cancer: a multi-institutional study. J Thorac Cardiovasc Surg 2006;132:507-12. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Belgers EH, Siebenga J, Bosch AM, et al. Complete video-assisted thoracoscopic surgery lobectomy and its learning curve. A single center study introducing the technique in The Netherlands. Interact Cardiovasc Thorac Surg 2010;10:176-80. [Crossref] [PubMed]
- Nomori H, Horio H, Suemasu K. Anterior limited thoracotomy with intrathoracic illumination for lung cancer: its advantages over anteroaxillary and posterolateral thoracotomy. Chest 1999;115:874-80. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. Available online: https://www.nccn.org/
- Jensen K, Petersen RH, Hansen HJ. Video-Assisted Thoracic Surgery Lobectomy: Review of Data Strongly Suggests the Interest of its Further Implementation. European Journal of Clinical & Medical Oncology 2011;3:26.
- Fiorelli A, Morgillo F, Milione R, et al. Control of post-thoracotomy pain by transcutaneous electrical nerve stimulation: effect on serum cytokine levels, visual analogue scale, pulmonary function and medication. Eur J Cardiothorac Surg 2012;41:861-8; discussion 868. [Crossref] [PubMed]
- Lim E, Batchelor TJP, Dunning J, et al. Video-Assisted Thoracoscopic or Open Lobectomy in Early-Stage Lung Cancer. NEJM Evid 2022;1:EVIDoa2100016.
- Fiorelli A, Santini M. In lung cancer patients where a malignant pleural effusion is found at operation could resection ever still be justified? Interact Cardiovasc Thorac Surg 2013;17:407-12. [Crossref] [PubMed]
- Krasna MJ, Deshmukh S, McLaughlin JS. Complications of thoracoscopy. Ann Thorac Surg 1996;61:1066-9. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [Crossref] [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Hireche K, Moqaddam M, Lonjon N, et al. Combined video-assisted thoracoscopy surgery and posterior midline incision for en bloc resection of non-small-cell lung cancer invading the spine. Interact Cardiovasc Thorac Surg 2022;34:74-80. [Crossref] [PubMed]
- Kawai N, Kawaguchi T, Yasukawa M, et al. Less Invasive Approach to Pancoast Tumor in a Partitioned Incision. Ann Thorac Cardiovasc Surg 2017;23:161-3. [Crossref] [PubMed]
- Burfeind WR, D’Amico TA. Thoracoscopic lobectomy. Op Tech Thorac Cardiovasc Surg 2004;9:98-114. [Crossref]
- Agasthian T. Initial experience with video-assisted thoracoscopic bronchoplasty. Eur J Cardiothorac Surg 2013;44:616-23. [Crossref] [PubMed]
- Li Y, Wang J. Video-assisted thoracoscopic surgery sleeve lobectomy with bronchoplasty: an improved operative technique. Eur J Cardiothorac Surg 2013;44:1108-12. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Uniportal video-assisted thoracoscopic sleeve lobectomy and other complex resections. J Thorac Dis 2014;6:S674-81. [PubMed]
Cite this article as: Francis R, Goldstein AL, Akar FA. Pancoast tumors. Video-assist Thorac Surg 2024;9:7.