Uniportal video-assisted thoracoscopic surgery (U-VATS) bronchial and vascular sleeve resections: technical aspects and review
Introduction
Since Roviaro et al. performed the first video-assisted thoracoscopic surgery (VATS) pulmonary lobectomy in the early 1990s (1), more than 10 years of scientific evidence were needed to be recognised as the standard surgical approach for early-stage lung cancer (2). Advantages over open thoracotomy include better recovery of preoperative physical function, less pain, shorter postoperative length of stay and reduced morbidity (3). As surgeons have gained experience with this approach, more complex cases have been performed reflecting the instinct of thoracic surgeons to push the established limits without compromising the safety and quality of oncological resections (4).
In the early 2000s, Rocco et al. popularised the uniportal VATS (U-VATS) approach, a modification of the VATS technique in which minor procedures such as bullectomies or wedge lung resections were completed using only a mini-thoracotomy (5). Gonzalez et al. published in 2011 the first U-VATS lung lobectomy (6), but few years later they broke down the barriers regarding the indication of this approach by successfully performing the first bronchial sleeve lobectomy as well as the first double (bronchial and arterial) sleeve lobectomy (7,8). Although enthusiasm with this technique has grown, studies with sufficient evidence, beyond institutional retrospective reviews, should be developed to validate its feasibility and safety in complex lung cancer surgery with the same oncological principles and similar outcomes compared to multiportal VATS or thoracotomy (4).
Although bronchovascular resection and reconstruction techniques in centrally located lung cancer have been extensively described in thoracotomies (9), the performance of these techniques by U-VATS requires the development and standardisation of specific surgical skills that facilitate and enable the safe reproduction of the procedure as when conducted by multiportal VATS or open surgery (10). In addition, a multidisciplinary surgical team experienced in U-VATS in a high-volume centre is of paramount importance in these complex patients to minimize risk of conversion, pneumonectomy or unnecessary exploratory thoracotomies. Despite U-VATS sleeve bronchovascular resections potential, the consensus statement of the Uniportal VATS Interest Group (UVIG) of the European Society of Thoracic Surgeons (ESTS) published in 2019, central tumour location involving hilar structures represented a relative contraindication for U-VATS resection in 61% of the responses and was not a contraindication in 32% (11).
The aim of this article is to describe the clinical indications and standardise the technical aspects of U-VATS bronchial and vascular sleeve resections in centrally located lung tumours, in order to simplify decision-making during surgery and avoid complications, as well as to review their results compared to pneumonectomy and the classic thoracotomy approach.
Definition and indications
Bronchial and vascular sleeve resections are completely circumferential resections of the involved bronchi or arteries followed by subsequent reconstruction by end-to-end anastomosis. They constitute a major challenge in themselves because they are probably the most complex interventions that can be carried out by U-VATS and require not only extensive prior experience of the surgical team in this approach, but also in bronchoplasties and angioplasties by thoracotomy (4,12).
It is estimated that up to 19% of lung cancer patients with a centrally located tumour will require a sleeve lobectomy (13). Bronchial and vascular reconstruction techniques, when technically and oncologically feasible, are recommended for the treatment of centrally located tumours or malignant infiltrative nodes when they involve the lobar bronchial carina, lobar arterial branches or extend into the main stem bronchus or main pulmonary artery (PA) to avoid an elective pneumonectomy (4,14). Potential advantages include less impairment in postoperative lung function, avoidance of potentially fatal complications associated with pneumonectomy such as bronchopleural fistula and postpneumonectomy pulmonary edema, preservation of right ventricular function and the option to surgically treat a relapse, as well as improvement in quality of life and cost-efficiency of the procedure (15).
Although any lobe is a possible candidate for bronchial sleeve resection, the most frequent location comprises lesions in the hilum of the right upper lobe (RUL), due to the anatomical layout of the right main stem and intermedius bronchi (16,17). Next in frequency are tumours located in the hilum of the left upper lobe (LUL), with or without extension to the left main stem bronchus. If PA involvement is also present, which is common in this location, a combined bronchial and vascular sleeve resection is required (10).
In general, despite negative imaging studies by computed tomography (CT) or positron emission tomography (PET), all patients with a central non-small cell lung cancer (NSCLC) require at least minimally invasive mediastinal staging by means of endobronchial ultrasound-transbronchial needle aspiration (EBUS-TBNA) to exclude unexpected N2 involvement, present in up to 21.6% of cases (18).
Pre-operative planning
The acquisition of a millimetric-thin sliced contrast-enhanced CT of the chest is of great value when planning the surgical strategy for a sleeve resection. It clearly reveals the location of the tumour and its relationship with hilar and mediastinal bronchovascular structures, as well as the extent of their infiltration. The study of its three-dimensional (3D) (axial, coronal, and sagittal) together with the possibility of creating a 3D reconstruction with the appropriate software, facilitates pre-operative planning and guides surgical sequence.
Occasionally, bronchovascular resection and reconstruction techniques may not be performed, mainly due to intraoperative findings, leading to the need for pneumonectomy. Therefore, patients should be precisely selected for these procedures according to their cardiopulmonary reserve to avoid unnecessary morbidity and mortality.
Bronchial airway should be directly assessed by the surgical team through a bronchoscopy to identify intraluminal involvement of the tumour and to clarify proximal and distal margins for sleeve resection and subsequent reconstruction.
The use of a 30° thoracoscope is mandatory in this type of procedure as well as specific instruments for minimally invasive surgery.
Anaesthesia, patient position and incision
Surgery is performed under general anaesthesia and orotracheal intubation, using a double-lumen tube (usually left-sided) or alternatively a bronchial blocker. Patient is positioned in lateral decubitus. In cases where left pneumonectomy may be considered, a right-sided tube is the best option. Sleeve resections have also been described, in very selected cases, in non-intubated patients (19).
Incision is about 4–5 cm in length and usually located in the 5th intercostal space around mid-axillary line. This placement allows the best angle for the handling of endoscopic staplers, especially in the left side for the division of the superior pulmonary vein. Depending on patient’s anatomy and tumour characteristics, an alternative in RUL sleeve lobectomies would be the 4th intercostal space, to target bronchial or arterial anastomosis from a more perpendicular position (20). Anterior or posterior rotation of the operating table may improve exposure when suturing the far or near edge of the anastomosis respectively (17). Camera should always be placed at the top of the incision so the rest of instruments should be introduced through the lower part, except in the eventual assessment of hilar structures during fissure division with endoscopic staplers.
Bronchial sleeve resections
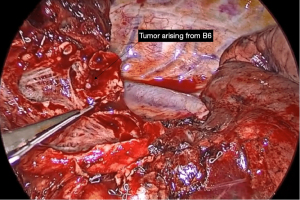
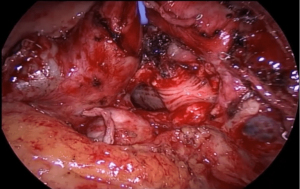
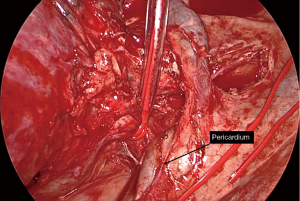
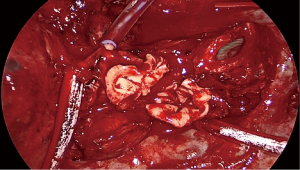
Not all tumours in which the need for a sleeve resection is considered preoperatively will ultimately require it. Its viability must be definitively assessed during the surgical procedure, considering the involvement of the tumour and lymph nodes proximally in the hilum and distally in the fissure, and deciding whether reconstruction of the defect can be achieved by a wide bronchial wedge (Figures 1,2) or by a sleeve.
Cases in which a sleeve lobectomy is finally necessary usually exhibit some common characteristics that increase complexity of an already extremely difficult procedure and compromise access to hilar structures: (I) centrality and size of the tumour; (II) potentially difficult access to the hilum due to infiltrated lymph nodes; (III) fibrosis accompanying inflammatory processes derived from the mass effect of the lesion or from previous administration of neoadjuvant treatments; and (IV) air trapping secondary to bronchial obstruction (17).
In any case, U-VATS sleeve resections in NSCLC must be performed under strict oncological principles identical to those achieved by the “traditional” thoracotomy approach: (I) negative proximal and distal bronchial margins confirmed intraoperatively by frozen section [for NSCLC a margin of at least 5 mm is ideally considered, although 3 mm is sufficient in less malignant tumours such as carcinoids (21)]; (II) en bloc resection of the surgical specimen; (III) proper accommodation of the anastomotic bronchial edges, suturing mucosa to mucosa avoiding diameter discrepancies; (IV) tension-free end-to-end anastomosis; and (V) systematic lymph node dissection (4,16).
Performing a video-assisted mediastinoscopy or a video-assisted mediastinoscopic lymphadenectomy (VAMLA) prior to resection provides pathological mediastinal staging as well as complete release of the trachea and main stem bronchus, which will reduce proximal tension to the anastomosis. It is inadmissible to perform it in a previous surgical session because the generation of adhesions and secondary hilar fibrosis would affect the safety of the procedure on the day of resection (4).
Surgical technique
Bronchial sleeve lobectomy is conducted as a standard lobectomy keeping the dissection of the bronchus for last. Under bronchoscopic assessment, proximal and distal bronchial margins are transected and surgical specimen is removed. Lymphadenectomy of 2R, 4R, and 10R territories on the right; 4L, 5, 6, and 10L on the left as well as territory 7 in both sides must be completed before starting the bronchial anastomosis, mainly to facilitate complete mobilization of the bronchial tree and reduce tension on the anastomosis and to check lymph node status rigorously. Ascent of residual parenchyma is favoured by the release of the pulmonary ligament and, in cases of larger defects, by a U-shaped pericardial opening around inferior pulmonary vein, anteriorly towards the PA and posteriorly to the main stem bronchus (Video 1).
U-VATS bronchial sleeve reconstruction should always be considered, after generating a reconstructable defect, with the intention to obtain a tension-free anastomosis without air leaks, to preserve bronchial vascularization and to ensure that knots are tied outside the bronchial lumen (4). If these circumstances are not met, it will be necessary to extend initially planned resection and avoid potential morbidity.
Several techniques and suture materials have been described proving the same effectiveness, reproducibility and results in clinical practice (interrupted, running suture or combinations; absorbable or non-absorbable threads). However, interrupted suture we are familiar with from open surgery, although technically feasible, is not suitable for U-VATS approach so most surgeons have adopted either one single or two separate threads running suture. It simplifies the procedure, avoids the tendency of threads to get snagged and tangled and saves operating time. The most commonly used materials are absorbable polydioxanone monofilament and non-absorbable polypropylene monofilament, with which no increased incidence of granulomas has been observed (22). Barbed sutures are worth mentioning too, as they maintain tension without the need for knot tying and achieve comparable results. The 3-0 size is the most frequently employed although 4-0 may be useful in more distal reconstructions.
The idea is that the membranous portion of both bronchial edges should always be oriented towards the posterior parietal pleura to avoid rotation of the anastomosis. This enables the suture to be started at the posterior and inferior junction of the membranous portion with the bronchial cartilages (Video 2), generally and as the preference of the authors, with a 90 cm long thread and two 17 mm 1/2c needles inside the bronchial lumen and to be finished at the anterior aspect of the anastomosis closest to the surgeon with knots tied extraluminally. By this way, a circumferential suture of 360° is achieved (Videos 3,4). Discrepancies in bronchial diameters are best managed by spacing the sutures at the proximal edge and bringing them together at the distal one. Alternatively, a small wedge resection can be made in the cartilaginous part of the larger bronchus to reshape its diameter or a bevelled section of the smaller bronchus to increase its circumference.
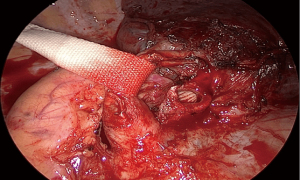
In right-sided bronchial sleeves a tourniquet can be placed on the proximal PA to compress it and close off blood flow, which facilitates exposure of the edges of the bronchial anastomosis located posterior to the artery (17). In more proximal ones, azygos vein can be divided if necessary. On the left side, superior and posterior traction of the PA by means of a vessel loop, tape or silk sutured to the parietal pleura increases the working space for the same purpose (10) (Figure 3).
Finally, the presence of suture-related air leaks must be visually checked under two-lung ventilation water test. This would result in the need for repair to achieve a sealed suture as an essential goal. A new bronchoscopic control at this point is mandatory to check the caliber and disposition of the anastomosis as well as to aspirate secretions and haematic debris that may impede lung expansion and generate complications in the early postoperative period. Suture buttressing with vitalised tissue such as pericardial fat, pleura, pericardium or intercostal muscle may be recommended, especially after induction treatments or extensive resections with increased risk of dehiscence. It is not clear if it prevents fistulae, but it may help resolve small air leaks. In cases of double bronchial and arterial sleeve reconstructions, it provides a physical barrier between both anastomoses.
Most suture-related complications depend on the interruption of bronchial vascularization. Division of bronchial arteries is compensated by the rich anastomotic network between the pulmonary and bronchial circulation, which accounts for 75–90% of bronchial blood supply at the level of the lobar bronchi (23). However, excessive devascularization may result in ischaemia, inadequate healing and anastomotic-related problems such as dehiscence, fistulae or stenosis. Therefore, extensive skeletonization of the bronchial tree should be avoided without compromising the thoroughness of lymph node dissection (24).
Tension at the anastomosis is another cause of potential complications so adequate mobilisation of the distal lobe(s) must be achieved throughout the manoeuvres previously mentioned to avoid this situation.
Retention of secretions and postoperative atelectasis are easily managed with standard measures, as opposed to potentially fatal complications such as bronchopleural fistula or anastomotic dehiscence. Depending on the clinical situation of the patient and the size of the defect, if they cannot be treated by interventional bronchoscopy or conservative treatment, reintervention by U-VATS, multiportal VATS or thoracotomy may be necessary according to the experience of the surgical team and the chances of success.
Vascular sleeve resections
Occasionally, in addition to the defining characteristics of tumours requiring bronchial sleeve resections described above, the PA is also involved. Consequently, as well as bronchial techniques, resection and subsequent vascular sleeve reconstruction is mandatory to preserve pulmonary parenchyma and avoid pneumonectomy. This is the case when invasion of the main PA is present, not only at the origin of one or more of its lobar branches, but reaching an extension that is generally greater than 50% of the circumference of the vessel, preventing its reconstruction by direct suture or by the use of a patch. Furthermore, feasibility of the procedure will be confirmed by the accessibility to the distal artery in the fissure and by the existence of sufficient proximal margin to permit vascular control manoeuvres to be carried out.
The most frequent location of tumours requiring vascular sleeve or double sleeve (bronchial and vascular) resection is the LUL because left PA surrounds the lobar bronchus along most of its length. On the right side, they are much less frequent due to the anterior location of the PA in relation to the RUL and intermedius bronchi, although much more complex because of the anatomical disposition of the superior vena cava (SVC), which makes the proximal control of PA in depth considerably more difficult. Moreover, the absence of the ligamentum arteriosum and the presence of the middle lobe also contribute to that complexity.
Vascular control
The key point when facing a vascular or a double bronchial and vascular sleeve procedure is proper vascular control. These manoeuvres will also help to control unexpected accidental bleeding during arterial dissection. The use of vascular clamps, although feasible, is less advisable in U-VATS because they take up unnecessary space when externalized through the incision, make instrumentation more difficult when completing the suture, impede removal of surgical specimen and are at risk of displacement. Deployable endoscopic bulldogs also have this risk, especially proximally, and facilitate tangling of suture threads. Intracavitary tourniquets secured by a silk (11) or a vessel loop (locked by two transverse polymeric clips to maintain tension) are the best alternative. Their advantages include more available space at the incision, difficulty of displacement and possibility of partial release and repositioning in case of bleeding after the anastomosis. If very proximal control is necessary because there is a narrow margin to the defect to be reconstructed, two tourniquets can be placed for greater security or, alternatively, a vascular clamp can be used externalized outside the wound retractor (Figure 4).
Surgical technique
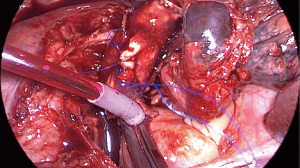
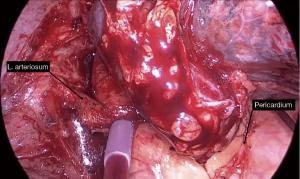
In experience of the authors, proximal vascular control of the intrapericardial PA through a wide pericardial incision posterior to the phrenic nerve is almost always recommended in order to obtain a greater distance to the tumour and minimize folding of the artery in the proximal stump. On the left side, proximal intracavitary tourniquet should be placed proximally to the ligamentum arteriosum, an anatomical structure that combined with the pericardial lamina will prevent displacement (Figure 5). If proximal tumour involvement makes it possible, proximal extrapericardial control is also feasible. On the right side, the absence of the ligamentum arteriosum makes it necessary to divide the azygos vein close to the SVC to facilitate its anterior traction, providing more proximal control of the PA (Figure 6). Distal control in the fissure requires its prior division to access the lower lobe arterial branches. If the fissure is partially invaded by the tumour, independent control of A6 and the basal trunk is also possible by placing two independent tourniquets. On the right side, distal control of the PA can be performed in the major fissure or more proximally in the minor one if bilobectomy is not required.
Intravenous heparin in doses of 1,500–5,000 IU is recommended prior to interruption of arterial blood flow to prevent thrombus formation and subsequent dislodgement during reperfusion as pulmonary embolism (25).
In double sleeve cases arteriotomy usually precedes the bronchial section, whereas in reconstructions the order is inverted to reduce tension in the vascular anastomosis. In addition, the absence of the resected arterial segment improves exposure to perform bronchial anastomosis in both hemithorax (Video 5, Figure 7).
If only an arterial sleeve resection is required, anastomosis is technically feasible in the presence of bronchial structures (Video 6, Figure 8). If arterial invasion is so extensive as to jeopardize a tension-free end-to-end anastomosis, especially in patients without bronchial sleeve resection, it is advisable to proceed by using a tubular pericardial graft or other material depending on surgical preferences. In left procedures, division of the ligamentum arteriosum may provide additional mobilisation of the proximal stump by more than 1 cm (26).
Technique for vascular anastomosis is similar to that described for bronchial anastomosis, except for the use of a smaller thread size (4-0 or 5-0). The preferred material is non-absorbable polypropylene monofilament, although as an alternative polytetrafluoroethylene (PTFE) offers better handling conditions in terms of flexibility. Knotting must be done taking care to avoid unnecessary traction that could tear the arterial wall and compromise its tightness. Before knotting, distal tourniquet must first be relaxed to encourage retrograde flow and remove air from the anastomosis to prevent air embolism. Finally, proximal tourniquet is partially released to re-establish pulmonary blood flow. If a bleeding event is identified at this suturing step, partially released proximal tourniquet (if a vessel loop has been used) can be repositioned for the repair, usually with an interrupted single stitch.
There are certain factors that may condition the U-VATS approach when performing a vascular sleeve or a double bronchial and vascular sleeve lobectomy even requiring, in experience of the authors, conversion to open surgery: (I) large tumours (T3–T4) that make lung manipulation and hilar exposure extremely difficult; (II) extensive involvement of the PA (>2 cm) that determines the need for reconstruction by means of a conduit or a patch; (III) excessive time-consuming manoeuvres during surgery, especially at the time of the anastomosis; and (IV) presence of hilar lymph nodes (calcified, bulky tumour involvement) that make vascular control nearly impossible. Ultimately, indication for thoracotomy will depend on the experience of each surgical team.
Evidence and discussion
Current trend among modern thoracic surgeons suggests that lung parenchyma-sparing techniques, when feasible and indicated to avoid pneumonectomy, are the best option in selected patients regardless lung functional status (14,15). Arguments in favour include better residual pulmonary function, lower short- and long-term surgical morbidity and mortality as well as a better quality of life without compromising oncological prognosis (14,27-29). This applies to the results of both bronchial and bronchovascular sleeves when compared to those of pneumonectomy (30). In a national retrospective study of 6,259 patients included in the French Epithor database published in 2017, Pagès et al. analysed 941 sleeve lobectomies compared to 5,318 pneumonectomies for NSCLC between 2005 and 2014. After propensity score matching between groups, 794 pairs of patients were defined. Both 3-year overall survival (OS) [71.86% vs. 60.76%; hazard ratio (HR): 1.63; 95% confidence interval (CI): 1.19–2.21] and disease-free survival (46.41% vs. 31.63%; HR: 1.49; 95% CI: 1.1–2) were slightly higher in sleeve lobectomy group compared to pneumonectomy group. Sleeve group also showed favourable results in terms of incidence of bronchopleural fistula, empyema and postoperative arrhythmias but unfavourable results related to atelectasis and pneumonia. There was no difference in postoperative mortality and the main limitation of the study was loss of data during follow-up (31). Abdelsattar et al. revised data from 23,964 patients registered in the United States’ National Cancer Database (NCDB) who underwent surgery for NSCLC or carcinoid tumours between 1998 and 2012. In total, 1,713 underwent sleeve lobectomy and 22,251 underwent pneumonectomy. After propensity score matching, results confirmed the findings of the French study regarding significant improvement in OS favouring sleeve lobectomy group during the first 18 months. Furthermore, in pneumonectomy group, both 30-day mortality (5.9% vs. 1.6%, P<0.001) and 90-day mortality (9.4% vs. 4%, P<0.001) were higher compared to sleeve group (32).
Since the publication of the first U-VATS bronchial sleeve lobectomy and double sleeve lobectomy in 2013 (7) and 2014 respectively (8), numerous authors have retrospectively reported their experience in an attempt to demonstrate the safety and feasibility of both techniques (33-36). The series with the largest number of cases was published in 2019 by Soultanis et al., describing 79 consecutive patients operated at Shanghai Pulmonary Hospital between 2014 and 2018 (37). Despite promising results, there are currently very few studies that provide sufficient level of evidence to compare these techniques by means of U-VATS or multiportal VATS vs. thoracotomy in terms of perioperative outcomes and long-term survival in NSCLC (37-41). Therefore, official clinical guidelines have not yet incorporated VATS sleeve bronchoplastic resections into their clinical practice recommendations (13). The systematic review and meta-analysis published by Geropoulos et al. in 2022 finally analysed 6 heterogeneous studies with non-overlapping populations that included 655 patients with NSCLC undergoing sleeve lobectomy (229 VATS vs. 426 open). Minimally invasive sleeve lobectomy was significantly associated with longer operative time [weighted mean difference (WMD): 45.85 minutes; 95% CI: 12.06–79.65; P=0.01] but reduced intraoperative blood loss (WMD: −34.57 mL; 95% CI: −58.35 to −10.78; P<0.001). No further differences were found in margin-negative resection rate, number of lymph nodes resected, postoperative outcomes (drainage duration, length of hospital stay and 30-day mortality), postoperative complications and long-term outcomes (OS and recurrence-free survival). Authors concluded that VATS approach was a feasible and safe alternative to open surgery for the treatment of centrally located NSCLC undergoing sleeve lobectomy (13).
Based on the results we have analysed thoracic surgeons have currently adopted, and will continue to do so in the future, minimally invasive approaches (specifically U-VATS) for the treatment of centrally located NSCLC when a sleeve lobectomy is required. The aim is to combine the benefits of these approaches with those from lung parenchyma-sparing surgery, offering the highest quality in this kind of procedures.
Conclusions
Natural instinct of thoracic surgeons to push surgical limits without compromising safety and quality of oncological resections has led to the adoption of minimally invasive approaches, in particular U-VATS, whose advantages combine with those from parenchyma-sparing surgery to offer the highest quality in bronchial and vascular sleeve lobectomy procedures for the treatment of centrally located NSCLC. Considering the results analysed in comparison to pneumonectomy and open thoracotomy, these techniques are safe, reproducible and standardizable although, due to the level of difficulty, they require a multidisciplinary surgical team with extensive previous experience in both U-VATS and broncho-angioplastic procedures.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Video-Assisted Thoracic Surgery for the series “Advanced Uniportal VATS”. The article has undergone external peer review.
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-23-44/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-23-44/coif). The series “Advanced Uniportal VATS” was commissioned by the editorial office without any funding or sponsorship. C.G. served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of our institutional research committee without further analysis as all were done following our standard practice. The study was conducted in accordance with the Helsinki Declaration (as revised in 2013) and written informed consent was routinely obtained from the patients for the publication of this article, accompanying images and videos.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. Non-small cell lung cancer, version 1.2015. J Natl Compr Canc Netw 2014;12:1738-61. [Crossref] [PubMed]
- Lim E, Batchelor TJP, Dunning J, et al. Video-assisted thoracoscopic or open lobectomy in early-stage lung cancer. NEJM Evid 2022;1:EVIDoa2100016.
- Royo-Crespo I, Vieira A, Ugalde PA. Extended uniportal video-assisted thoracic surgery for lung cancer: is it feasible? J Vis Surg 2018;4:57. [Crossref] [PubMed]
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [Crossref] [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Uniportal video-assisted thoracoscopic bronchial sleeve lobectomy: first report. J Thorac Cardiovasc Surg 2013;145:1676-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Double sleeve uniportal video-assisted thoracoscopic lobectomy for non-small cell lung cancer. Ann Cardiothorac Surg 2014;3:E2. [PubMed]
- Yamashita M, Komori E, Sawada S, et al. Pulmonary angioplastic procedure for lung cancer surgery. Gen Thorac Cardiovasc Surg 2010;58:19-24. [Crossref] [PubMed]
- Sesma J, Bolufer S, Galvez C, et al. Technical aspects of uniportal VATS lung major resections with bronchovascular reconstruction. Chin J Thorac Surg 2021;8:209-17. (Electronic Edition).
- Bertolaccini L, Batirel H, Brunelli A, et al. Uniportal video-assisted thoracic surgery lobectomy: a consensus report from the Uniportal VATS Interest Group (UVIG) of the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;56:224-9. [Crossref] [PubMed]
- Gonzalez-Rivas D, Garcia A, Chen C, et al. Technical aspects of uniportal video-assisted thoracoscopic double sleeve bronchovascular resections. Eur J Cardiothorac Surg 2020;58:i14-22. [Crossref] [PubMed]
- Geropoulos G, Esagian SM, Skarentzos K, et al. Video-assisted thoracoscopic versus open sleeve lobectomy for non-small cell lung cancer: A systematic review and meta-analysis from six comparative studies. Asian Cardiovasc Thorac Ann 2022;30:881-93. [Crossref] [PubMed]
- Ma Z, Dong A, Fan J, et al. Does sleeve lobectomy concomitant with or without pulmonary artery reconstruction (double sleeve) have favorable results for non-small cell lung cancer compared with pneumonectomy? A meta-analysis. Eur J Cardiothorac Surg 2007;32:20-8. [Crossref] [PubMed]
- Martin-Ucar AE, Chaudhuri N, Edwards JG, et al. Can pneumonectomy for non-small cell lung cancer be avoided? An audit of parenchymal sparing lung surgery. Eur J Cardiothorac Surg 2002;21:601-5. [Crossref] [PubMed]
- Davoli F, Bertolaccini L, Pardolesi A, et al. Video-assisted thoracoscopic surgery bronchial sleeve lobectomy. J Vis Surg 2017;3:41. [Crossref] [PubMed]
- Soultanis KM, Gonzalez-Rivas D. Uniportal video-assisted sleeve resections: how to deal with specific challenges. J Thorac Dis 2019;11:S1670-7. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Shao W, Phan K, Guo X, et al. Non-intubated complete thoracoscopic bronchial sleeve resection for central lung cancer. J Thorac Dis 2014;6:1485-8. [PubMed]
- Sekhniaidze D, Agasiev M, Obuhova T. Uniportal sleeve lobectomy for lung cancer. J Vis Surg 2017;3:159. [Crossref] [PubMed]
- Predina JD, Kunkala M, Aliperti LA, et al. Sleeve lobectomy: current indications and future directions. Ann Thorac Cardiovasc Surg 2010;16:310-8. [PubMed]
- Kocher GJ. Multiport and uniportal VATS sleeve resections. J Thorac Dis 2019;11:1091-3. [Crossref] [PubMed]
- Fréchette E, Deslauriers J. Surgical anatomy of the bronchial tree and pulmonary artery. Semin Thorac Cardiovasc Surg 2006;18:77-84. [Crossref] [PubMed]
- Faber LP, Jensik RJ, Kittle CF. Results of sleeve lobectomy for bronchogenic carcinoma in 101 patients. Ann Thorac Surg 1984;37:279-85. [Crossref] [PubMed]
- Maurizi G, D'Andrilli A, Venuta F, et al. Bronchial and arterial sleeve resection for centrally-located lung cancers. J Thorac Dis 2016;8:S872-81. [Crossref] [PubMed]
- Lyscov A, Obukhova T, Ryabova V, et al. Double-sleeve and carinal resections using the uniportal VATS technique: a single centre experience. J Thorac Dis 2016;8:S235-41. [PubMed]
- Stallard J, Loberg A, Dunning J, et al. Is a sleeve lobectomy significantly better than a pneumonectomy? Interact Cardiovasc Thorac Surg 2010;11:660-6. [Crossref] [PubMed]
- Maurizi G, D'Andrilli A, Anile M, et al. Sleeve lobectomy compared with pneumonectomy after induction therapy for non-small-cell lung cancer. J Thorac Oncol 2013;8:637-43. [Crossref] [PubMed]
- Deslauriers J, Grégoire J, Jacques LF, et al. Sleeve lobectomy versus pneumonectomy for lung cancer: a comparative analysis of survival and sites or recurrences. Ann Thorac Surg 2004;77:1152-6; discussion 1156. [Crossref] [PubMed]
- Lausberg HF, Graeter TP, Tscholl D, et al. Bronchovascular versus bronchial sleeve resection for central lung tumors. Ann Thorac Surg 2005;79:1147-52; discussion 1147-52. [Crossref] [PubMed]
- Pagès PB, Mordant P, Renaud S, et al. Sleeve lobectomy may provide better outcomes than pneumonectomy for non-small cell lung cancer. A decade in a nationwide study. J Thorac Cardiovasc Surg 2017;153:184-195.e3. [Crossref] [PubMed]
- Abdelsattar ZM, Shen KR, Yendamuri S, et al. Outcomes After Sleeve Lung Resections Versus Pneumonectomy in the United States. Ann Thorac Surg 2017;104:1656-64. [Crossref] [PubMed]
- Koryllos A, Stoelben E. Uniportal video-assisted thoracoscopic surgery (VATS) sleeve resections for non-small cell lung cancer patients: an observational prospective study and technique analysis. J Vis Surg 2018;4:16. [Crossref] [PubMed]
- Qu JC, Soultanis KM, Jiang L. Surgical techniques and outcome analysis of uniportal video-assisted thoracic surgery complex sleeve lung resection: a 20 case-series study. J Thorac Dis 2021;13:2255-63. [Crossref] [PubMed]
- Caso R, Watson TJ, Khaitan PG, et al. Outcomes of minimally invasive sleeve resection. J Thorac Dis 2018;10:6653-9. [Crossref] [PubMed]
- Huang J, Li J, Qiu Y, et al. Thoracoscopic double sleeve lobectomy in 13 patients: a series report from multi-centers. J Thorac Dis 2015;7:834-42. [PubMed]
- Soultanis KM, Chen Chao M, Chen J, et al. Technique and outcomes of 79 consecutive uniportal video-assisted sleeve lobectomies. Eur J Cardiothorac Surg 2019;56:876-82. [Crossref] [PubMed]
- Mayne NR, Darling AJ, Raman V, et al. Perioperative Outcomes and 5-year Survival After Open versus Thoracoscopic Sleeve Resection for Lung Cancer. Semin Thorac Cardiovasc Surg 2021;33:522-30. [Crossref] [PubMed]
- Xie D, Deng J, Gonzalez-Rivas D, et al. Comparison of video-assisted thoracoscopic surgery with thoracotomy in bronchial sleeve lobectomy for centrally located non-small cell lung cancer. J Thorac Cardiovasc Surg 2021;161:403-13.e2. [Crossref] [PubMed]
- Yang Y, Mei J, Lin F, et al. Comparison of the Short- and Long-term Outcomes of Video-assisted Thoracoscopic Surgery versus Open Thoracotomy Bronchial Sleeve Lobectomy for Central Lung Cancer: A Retrospective Propensity Score Matched Cohort Study. Ann Surg Oncol 2020;27:4384-93. [Crossref] [PubMed]
- Wu L, Wang H, Cai H, et al. Comparison of Double Sleeve Lobectomy by Uniportal Video-Assisted Thoracic Surgery (VATS) and Thoracotomy for NSCLC Treatment. Cancer Manag Res 2019;11:10167-74. [Crossref] [PubMed]
Cite this article as: Bolufer S, Sesma J, Sebastian L, Maroto S, Vaillo X, Lirio F, del Campo J, Galvez C. Uniportal video-assisted thoracoscopic surgery (U-VATS) bronchial and vascular sleeve resections: technical aspects and review. Video-assist Thorac Surg 2024;9:6.