Surgical technique: lung-sparing sleeve bronchoplasty
Highlight box
Surgical highlights
• Lung-sparing sleeve bronchoplasty performed by uniportal video-assisted thoracoscopic surgery (VATS).
What is conventional and what is novel/modified?
• Lung-sparing bronchoplasty is usually performed via open thoracotomy due to the difficulty of the technique.
• Using a uniportal VATS technique is feasible and allows for the benefits of minimally invasive thoracic surgery, which include less pain and faster recovery.
What is the implication, and what should change now?
• A minimally invasive approach should be considered for centrally located tumors requiring a lung-sparing resection.
Introduction
Background
Surgery is the optimal treatment for low-grade centrally located tumors found in the bronchi, such as neuroendocrine tumors, mucoepidermoid carcinoma, and adenoid cystic carcinoma, and surgery may be part of the treatment of benign strictures, such as those found in patients with tuberculosis or endemic mycoses. Preserving the lung parenchyma while maintaining an oncologically sound resection when removing these tumors remains a challenge (1). Although more technically demanding than a lobectomy or pneumonectomy, numerous reports have shown that bronchoplasty with preservation of the parenchyma yields better outcomes than standard lung resection surgeries (2-4).
Rationale and knowledge gap
Advances in minimally invasive surgery and diagnostic methods have favored the development of thoracoscopic parenchyma-preserving surgical techniques. The feasibility of using uniportal, or single-port, video-assisted thoracoscopic surgery (VATS) to perform complex resections has been acknowledged in several series and case reports (5,6) with oncological results that are not inferior to multiportal VATS (7). Uniportal VATS parenchymal-sparing bronchoplasty can be considered by surgeons experienced with uniportal surgery and with VATS in general.
Objective
To demonstrate the technique of minimally invasive lung-parenchyma-sparing bronchoplasty, we present a lung-sparing sleeve resection and anastomosis in the left main bronchus using a uniportal approach. We present this article in accordance with the SUPER reporting checklist (available at https://vats.amegroups.com/article/view/10.21037/vats-23-19/rc).
Preoperative preparations and requirements
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript, the accompanying images and the video. A copy of the written consent is available for review by the editorial office of this journal.
Three factors should be taken in consideration when performing a lung-sparing bronchoplasty:
- Location. The bronchoplasty should be performed on a long bronchial segment, allowing for resection and proper reconstruction. The left main bronchus, intermedius bronchus, and the right main bronchus are typically good locations to perform a bronchoplasty.
- Etiology. Benign stenoses and well-differentiated tumors are the ideal types of lesions for this technique.
- Size. The smaller the segment affected is, the better the chances of success are. Resection of lesions or segments ≤2 cm is recommended to facilitate the re-anastomosis.
Two techniques are available:
- Anterior approach (described below). Straight forward from the uniportal point of view.
- Posterior approach. Performed via robotic surgery or multiportal VATS with the camera in the inferior port.
Preoperative bronchoscopy can provide information to plan the extent of the surgery and assess the viability of the lung to reinflate. Endoscopic laser treatment may be performed during preoperative bronchoscopy to restore the bronchial lumen and treat obstruction (8).
Surgical technique: step-by-step description
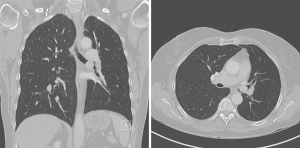
A 67-year-old male patient was diagnosed with an endobronchial typical carcinoid tumor at the level of the secondary carina in the left bronchus (Figure 1). The histopathological diagnosis was confirmed through flexible bronchoscopy, which was also used to assess whether the tumor was resectable. An oncological evaluation did not reveal distant metastasis, and a functional evaluation confirmed normal lung function allowing for pulmonary surgery, and even lung resection, if needed.
As per usual, and due to the level of difficulty of such case, this surgery was performed in the operating room at a tertiary hospital. The team for this procedure included a scrub nurse, a circulating nurse, the surgeon performing the procedure, a second surgeon or physician assistant and the anesthetist. The surgeon performing the procedure must have sufficient experience with complex thoracoscopic procedures.
The patient was placed under general anesthesia and intubated with a double-lumen tube. Videomediastinoscopy was performed, as is routine in our practice, to dissect and liberate the trachea and the left main bronchus to provide maximal mobilization and reduce tension during the procedure. A complete lymphadenectomy was also performed of stations 4D, 7, 4L and 10L for optimal mediastinal staging (Video 1).
After confirming that there were no signs of extrabronchial invasion or positive lymph nodes on frozen sections, the patient was placed in the right lateral decubitus position, and the bronchoplasty was started through a single 3–4 cm incision in the 5th intercostal space, between the anterior and midaxillary lines.
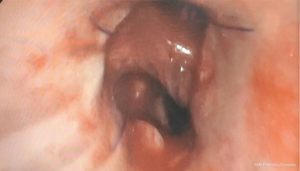
The inferior pulmonary ligament was divided for better mobilization. Both lobes of the left lung were retracted posteriorly, and the hilum was dissected anteriorly between the veins of the left upper lobe (LUL) and left lower lobe (LLL). Care was taken to avoid damaging the phrenic nerve. The lymph nodes of stations 10 and 11 were removed, allowing for the visualisation of the secondary carina. The pulmonary artery in the fissure was dissected away from the bronchus providing room for the sleeve bronchoplasty. The veins were also dissected away from the bronchial tree, using hook cautery and bipolar energy, allowing for full exposure of the left main bronchus and secondary carina (Figure 2). The fissure between the lobes was transected using an endostapler. Lymph node dissection was achieved according to international guidelines (9).
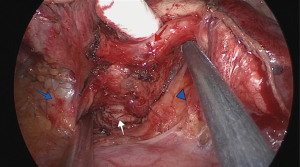
A perioperative bronchoscopy was performed, and proximal and distal bronchial margins were transected under thoracoscopic and endoscopic visualization (Figure 3). The specimen was sent for frozen-section analysis.
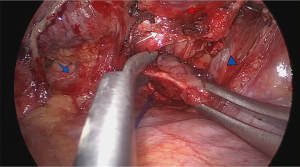
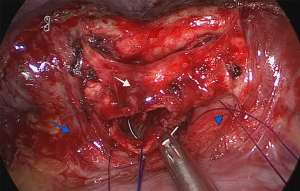
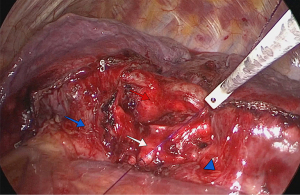
After the pathologist confirmed that the margins were free of neoplastic tissue, a running suture was performed with double-armed 4.0 polydioxanone suture (PDS 4.0). Care was taken to avoid tangling of the sutures. The anastomosis started in the membranous portion of the bronchi (posterior wall), with half performed clockwise and the other half performed counterclockwise with sutures placed 2 mm apart (Figure 4). The pulmonary artery and the superior and inferior lobe veins were protected using a 5-mm straight suction tool to retract the vessels. Next, both threads were attached together anteriorly with a knot pusher, while tension was placed to avoid a loose anastomosis (Figure 5).
Finally, we performed an air leak test by injecting 1 L of saline into the chest cavity followed by lung re-expansion under direct vision. The last step in the surgical procedure was the application of intercostal nerve blocks from the 2nd to the 8th intercostal spaces using bupivacaine or lidocaine. A 24-F chest tube was introduced through the incision used for the uniportal surgery (Video 1).
Postoperative considerations
A postoperative bronchoscopy was done to confirm patency of the left main bronchus and confirm a good visual aspect of the anastomosis (Figure 6). The length of the procedure was 200 minutes. Patient recovered in a stepdown unit for 24 hours and was transferred to the thoracic surgery ward afterwards. He was discharged on postoperative day 4.
A follow-up appointment was conducted 30 days postoperatively with a chest X-ray and bronchoscopy to visually examine aspects of the anastomosis.
Tips and pearls
- Performing videomediastinoscopy before doing the bronchoplasty facilitates dissection on the bronchus and reduces tension on the anastomosis.
- The inferior pulmonary ligament should be transected to reduce tension on the anastomosis.
- Dissecting the secondary carina and main bronchus away from the pulmonary artery is key for a successful bronchoplasty.
- Less is more. Using a single-running-suture technique reduces the chances of tangling the threads during the procedure.
- Always test the anastomosis by submerging the segment in saline and asking the anesthesia team to inflate the lung.
Discussion
The first report of bronchoplasty dates back to 1947 and was a report of a circumferential resection of an adenoma of the right main bronchus at the level of the right upper lobe bronchus, without any complications (10). A few years later, a series of 18 patients who underwent bronchoplasty for either a benign or malignant lesion was published by Paulson and Shaw, reinforcing the need to preserve lung function in patients with restricted lung capacity, particularly patients with chronic obstructive pulmonary disease (COPD) (11,12).
Gradually, the concept of lung-parenchyma-sparing bronchoplasty evolved and what was once a technique used only in patients with compromised lung capacity rapidly became accepted for many surgical candidates (13). Individuals once resigned to the side effects of a pneumonectomy saw hope in the fact that bronchial resections with complete lung preservation could be successful in selected patients. This technique is achievable in all bronchial segments but is commonly described in the right main bronchus, intermedius bronchus and the left main bronchus.
Low-grade and localized tumors and benign strictures are the ideal pathologies for lung-sparing bronchoplasty. Neoplasms must be biopsied before proceeding to resection to ensure that they are classified correctly histologically and that the peripheral margins will be negative to achieve an R0 resection. Benign strictures require proper investigation and treatment of the underlying cause in addition to resection. The affected segment must show no signs of active inflammation or granulation, otherwise, there will be an increased risk of recurrence.
Advances in minimally invasive techniques, with improved optics and dedicated video-assisted instruments, have allowed new approaches to resections that were commonly performed through thoracotomy due to their complexity. Surgeons in high-volume centers continue to innovate with new techniques and new indications for established techniques allowing complex surgeries to be performed minimally invasively. Uniportal video-assisted lobectomy was first described in 2010 (14), and was rapidly accepted and used worldwide. Reports on the learning curve of uniportal VATS (15) and demonstrating that advanced procedures were feasible through this approach inundated medical journals (5,16-18). Reports detailing uniportal lung-parenchyma-sparing bronchoplasty, although scarce due to the nature and complexity of the procedure, have been published confirming the feasibility and safety of the procedure when performed by well-trained surgeons (19,20).
There are two types of bronchoplasty to choose from when performing resection and anastomosis on a bronchial segment: wedge resection and sleeve resection. The wedge resection is a simpler option and involves removing a part of a bronchus wall, usually in a V-shape, and keeping the opposite wall intact. Although this approach facilitates creation of the anastomosis, because there is a smaller area to close and orientation is not a problem, it may cause increased tension in the anastomosis due to the intact back wall. The sleeve resection requires circumferential resection of the affected bronchus and is harder to reattach, because it requires 360º suturing, but sleeve resection allows for a more anatomical connection with less tension as compared with the wedge resection.
Another matter of debate is the optimal suturing technique for bronchoplasty. Several techniques have been described for traditional bronchoplasty performed by open thoracotomy (1,5). One option is to place interrupted sutures with PDS/Vicryl 4.0 (Ethicon Inc., Somerville, NJ, USA) with the knots tied outside the lumen. However, when performing video-assisted bronchoplasty, we prefer to use a running suture with double-armed/double-needled PDS 4.0 or Prolene 4.0 (Ethicon Inc., Somerville, NJ, USA). This running suture requires tying of the threads only once, which facilitates the procedure.
Although safer than more extensive surgeries with lung resection, lung-sparing bronchoplasty carries a significant risk of complications. In the acute postoperative phase, the most common complications are atelectasis caused by abundant bronchial secretions and persistent air leak. Other less-frequent complications are hemoptysis, hemothorax, and transient vocal cord paralysis depending on the location of the tumor and dissection performed. Late complications that have been reported are bronchial strictures, fistulas, dehiscence of the anastomosis, empyema and bronchiectasis, and recurrence of malignancy (21).
Conclusions
Uniportal lung-parenchyma-sparing bronchoplasty is an exceptional approach for resecting centrally located low-grade localized tumors and benign strictures in selected patients. Although advanced experience in VATS surgery is advised when undertaking this type of procedure, this technique has been proved to be safe and reproducible when a complete resection is possible.
Acknowledgments
We thank Shannon Wyszomierski, PhD for editing a draft of the article.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Video-Assisted Thoracic Surgery for the series “Advanced Uniportal VATS”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://vats.amegroups.com/article/view/10.21037/vats-23-19/rc
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-23-19/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-23-19/coif). The series “Advanced Uniportal VATS” was commissioned by the editorial office without any funding or sponsorship. P.U. served as the unpaid Guest Editor of the series. P.U. is a speaker of AstraZeneca, Medtronic, Takeda Pharmaceutical Company, and Merck & Co., and received institutional support from Harvard Medical School. A.V. reports that he receives honoraria for lectures from Bristol Myers Squibb (BMS) and F. Hoffmann-La Roche (Roche). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript, the accompanying images and the video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bueno R, Wain JC, Wright CD, et al. Bronchoplasty in the management of low-grade airway neoplasms and benign bronchial stenoses. Ann Thorac Surg 1996;62:824-8; discussion 828-9. [Crossref] [PubMed]
- Deslauriers J, Grégoire J, Jacques LF, et al. Sleeve lobectomy versus pneumonectomy for lung cancer: a comparative analysis of survival and sites or recurrences. Ann Thorac Surg 2004;77:1152-6; discussion 1156. [Crossref] [PubMed]
- Ludwig C, Stoelben E, Olschewski M, et al. Comparison of morbidity, 30-day mortality, and long-term survival after pneumonectomy and sleeve lobectomy for non-small cell lung carcinoma. Ann Thorac Surg 2005;79:968-73. [Crossref] [PubMed]
- Okada M, Yamagishi H, Satake S, et al. Survival related to lymph node involvement in lung cancer after sleeve lobectomy compared with pneumonectomy. J Thorac Cardiovasc Surg 2000;119:814-9. [Crossref] [PubMed]
- Royo-Crespo I, Vieira A, Ugalde PA. Extended uniportal video-assisted thoracic surgery for lung cancer: is it feasible? J Vis Surg 2018;4:57. [Crossref] [PubMed]
- Gonzalez-Rivas D, Soultanis KM, Garcia A, et al. Uniportal video-assisted thoracoscopic lung sparing tracheo-bronchial and carinal sleeve resections. J Thorac Dis 2020;12:6198-209. [Crossref] [PubMed]
- Bourdages-Pageau E, Vieira A, Lacasse Y, et al. Outcomes of Uniportal vs Multiportal Video-Assisted Thoracoscopic Lobectomy. Semin Thorac Cardiovasc Surg 2020;32:145-51. [Crossref] [PubMed]
- Dell'Amore A, Chen L, Monaci N, et al. Total Lung-sparing Surgery for Tracheobronchial Low-grade Malignancies. Ann Thorac Surg 2021;112:450-8. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 1.2023). Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed 01 February, 2023.
- THOMAS CP. Conservative resection of the bronchial tree. J R Coll Surg Edinb 1956;1:169-86. [PubMed]
- Paulson DL, Shaw RR. Preservation of lung tissue by means of bronchoplastic procedures. Am J Surg 1955;89:347-55. [Crossref] [PubMed]
- Paulson DL, Shaw RR. Results of bronchoplastic procedures for bronchogenic carcinoma. Ann Surg 1960;151:729-40. [Crossref] [PubMed]
- Vogt-Moykopf I, Fritz T, Meyer G, et al. Bronchoplastic and angioplastic operation in bronchial carcinoma: long-term results of a retrospective analysis from 1973 to 1983. Int Surg 1986;71:211-20. [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Vieira A, Bourdages-Pageau E, Kennedy K, et al. The learning curve on uniportal video-assisted thoracic surgery: An analysis of proficiency. J Thorac Cardiovasc Surg 2020;159:2487-2495.e2. [Crossref] [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Double sleeve uniportal video-assisted thoracoscopic lobectomy for non-small cell lung cancer. Ann Cardiothorac Surg 2014;3:E2. [PubMed]
- Gonzalez-Rivas D, Yang Y, Stupnik T, et al. Uniportal video-assisted thoracoscopic bronchovascular, tracheal and carinal sleeve resections†. Eur J Cardiothorac Surg 2016;49:i6-16. [PubMed]
- Guo M, Peng G, Wei B, et al. Uniportal video-assisted thoracoscopic surgery in tracheal tumour under spontaneous ventilation anaesthesia. Eur J Cardiothorac Surg 2017;52:392-4. [Crossref] [PubMed]
- AlShimali H, Rivas DG, Alshehab D, et al. Uniportal VATS Total Lung Sparing Right Main Bronchus Sleeve Resection With Secondary Carinal Reconstruction. CTSNet, Inc.; 2021.
- González-Rivas D, Garcia A, Chen C, et al. Technical aspects of uniportal video-assisted thoracoscopic sleeve resections: Where are the limits? JTCVS Tech 2020;2:160-4. [Crossref] [PubMed]
- Krüger M, Uschinsky K, Hässler K, et al. Postoperative complications after bronchoplastic procedures in the treatment of bronchial malignancies. Eur J Cardiothorac Surg 1998;14:46-52; discussion 52-3. [Crossref] [PubMed]
Cite this article as: Vieira A, Azevedo I, Ugalde P. Surgical technique: lung-sparing sleeve bronchoplasty. Video-assist Thorac Surg 2024;9:9.