
Uniportal video-assisted thoracic surgery for the creation of a total pleural covering for patients with Birt-Hogg-Dubé syndrome and secondary pneumothorax associated with hereditary multiple pulmonary cysts: surgical technique
Highlight box
Surgical highlights
• Total pleural covering (TPC) is a surgical procedure covering the entire visceral pleura on the surgical side using oxidized regenerative cellulose, which effectively prevents pneumothorax recurrence in patients of Birt-Hogg-Dubé syndrome.
• In uniportal video-assisted thoracic surgery (VATS) for TPC, the port is inserted on the sixth intercostal mid-axillary line.
What is conventional and what is novel/modified?
• Previously, 4-port VATS was necessary for toral pleural covering
• Now, total covering can be performed via uniport as same quality as 4-port.
What is the implication, and what should be change now?
• Uniportal VATS has the advantage of limiting the cause of postoperative pain to a single site, because there is only one intercostal nerve injury and one wound site. Therefore, we provide uniportal VATS for TPC as a minimally invasive surgery.
Introduction
Several diseases can lead to secondary pneumothorax arising from extensive and diffuse pulmonary cysts affecting the entire lung. Some are smoking-related, such as advanced-stage diffuse pulmonary emphysema; while others are genetically related, such as lymphangioleiomyomatosis (LAM), caused by mutations in the genes associated with tuberous sclerosis complex (TSC), and Birt-Hogg-Dubé syndrome (BHDS), which is associated with mutations in the folliculin (FCLN) gene. These genetic conditions are associated with multiple lung cysts that lead to refractory pneumothorax. Because the multiple lung cysts are widespread, they cannot all be removed surgically. We previously reported on a 4-port video-assisted thoracic surgery (VATS) procedure that created a total pleural covering (TPC) for the entire pleural viscera. It consisted of oxidized regenerative cellulose (ORC) mesh on the operated side. The covering successfully reduced the recurrence of postoperative pneumothorax in BHDS patients (1).
In primary spontaneous pneumothorax, which is more common in young men, the lung cysts are localized near the apices of the upper and lower lobes of the lung. Accordingly, resection, ablation, or looping of these areas, followed by a selective pleural covering of the regions of emphysematous change using ORC mesh, have been reported to be effective in randomized clinical trials (2). The effects of covering the visceral pleura with ORC mesh have been reported by Ebana and colleagues. After the covering has been placed, the thoracic cavity becomes an acidic environment. Approximately 12 hours later, the pH returns to neutral (data not submitted). These “acid ablation” conditions have led to a mesothelial-mesenchymal transition and decreased fibrinogenesis in human pleural mesothelial cells. These ultimately have resulted in an anti-adhesive state and thickening of the visceral pleura (3). The Gynecare Interceed Absorbable Adhesion Barrier (Jonson & Johnson, Brunswick, NJ, USA) has also recently been used as a synthetic absorbable anti-adhesive sheet for pleural coverings; however, at the time of this writing, we have primarily used ORC Mesh (Ethicon Surgicel Absorbable Hemostat gauze; Jonson & Johnson, Brunswick, NJ, USA) for pleural coverings because of its superior flexibility, our extensive experience with it, and our published work on it. The aim of this article was to provide a detailed description of a minimally invasive uniportal video-assisted thoracic surgery (U-VATS) procedure, which has the great advantage of limiting the cause of postoperative pain to a single site, that we have used to create a TPC with the use of ORC mesh. The procedure was used to treat patients with BHDS and secondary pneumothorax due to multiple pulmonary cysts.
Why TPC is necessary
We previously published the results of a study of patients with BHDS that compared covering only the regions of emphysematous change in the lungs with ORC mesh to a TPC with ORC mesh. TPC was the overwhelming winner (1). The following text describes the published data in detail. In patients with BHDS, lung cysts are found to occur on chest radiographs notably in the visceral pleura bordering the diaphragm, in the mediastinum, and in the interlobar space, especially in the middle and lower lung fields (4,5). Therefore, initially, pleural coverings were only applied to the common sites of pulmonary cysts, as follows: in the middle and the lower lobes with interlobar lesions in the right lung and in the lingual segment and the lower lobe with interlobar lesions in the left lung. This procedure was performed in 38 patients, and was called “lower pleural covering (LPC)”. The recurrence rates after the LPC procedure increased over time from 5.4% to 12% to 42% at 2.5, 5.0, and 7.5 years respectively (1). On the other hands, in 52 patients who underwent a TPC procedure covering the entire visceral pleura on the surgical side, the recurrence rates after the TPC procedure were 0%, 0%, and 0% at 2.5, 5.0, and 7.5 years, respectively. The recurrence rate also did not increase after an even longer follow-up period. Narrow-band imaging revealed numerous flat lung cysts along the interlobular septum, even in the right upper lobe and left superior segment, which used to be thought to have few lung cysts. These findings indicated that the extent of pleural covering by ORC mesh in patients with BHDS should be TPC instead of selective covering (1). To illustrate the surgical technique that is the purpose of this paper, we present a representative case in which U-VATS TPC was performed for a secondary pneumothorax in BHDS in the next paragraphs.
Representative case presentation for U-VATS TPC description
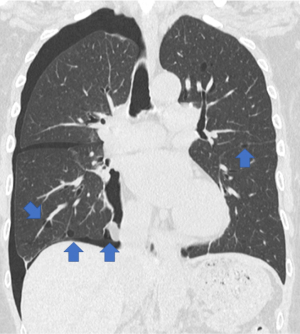
The patient was a 66-year-old woman with a definitive diagnosis of BHDS based on the modified Menko criteria for BHDS (6). Initially, she underwent a left 4-port VATS TPC at another hospital because she suffered from a recurring left secondary pneumothorax at 60 years of age. Thereafter, she had not developed postoperative recurrence of a left pneumothorax. This time, she was referred to our department for TPC surgery due to recurrent right secondary pneumothorax. Her chest computed tomography (CT) revealed a right pneumothorax and bilateral, multiple, and irregularly shaped cysts of various sizes (Figure 1). Since she had already been clinically diagnosed with BHDS, we selected and performed a U-VATS TPC procedure under general anesthesia and one-lung ventilation. We believe that this surgical technique is appropriate to demonstrate our current routine TPC procedure. Therefore, we would like to describe in detail the U-VATS TPC mentioned above at the next section. We present this article in accordance with the SUPER reporting checklist (available at https://vats.amegroups.com/article/view/10.21037/vats-23-48/rc).
Preoperative preparations and requirements
The operation was performed with the patient in the contralateral decubitus position and under general anesthesia and one-lung ventilation. The patient was fixed in the left lateral decubitus position, and a pillow was placed on the left side of the chest to make the right intercostal space as wide as possible. The surgical field was disinfected with iodine, and a cephazolin infusion was administered according to Centers for Disease Control and Prevention (CDC) guidelines. This procedure is contraindicated in cases of intra-thoracic infection, because of the placement of ORC mesh, which is a foreign body that remains in the thoracic cavity. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Regarding ethical issues, our local Ethical Review Committee determined that certification by the Ethical Committee and consent for publication from the patient were not necessary, because this paper contained a case report and a description of a surgical procedure that did not identify personal information.
Step-by step description
Surgical procedure
After an intercostal nerve block was performed as preemptive analgesia, an approximately 4-cm incision was created on the midaxillary line in the 6th intercostal space, and extending into the right thoracic cavity. An S-sized Alexis® O Wound Protector/Retractor (Applied Medical; Rancho Santa Margarita, CA, USA) was used to open the incision. In patients with BHDS, multiple pulmonary cysts are often found on the mediastinal side of the right middle lobe S5. Both a diagnostic and therapeutic partial lung resection of the S5 were performed for our patient by an automatic stapler. This site tends to be difficult to cover because of its pointed shape. We believe that a partial pulmonary resection can achieve smooth coverage (Figure 2).
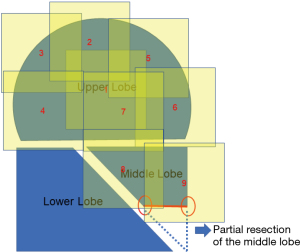
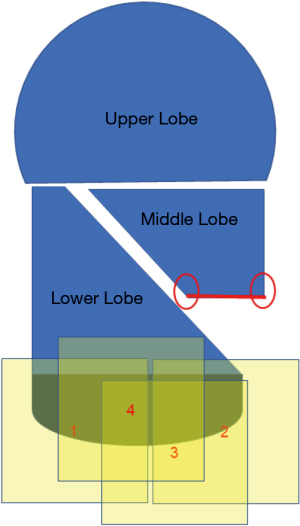
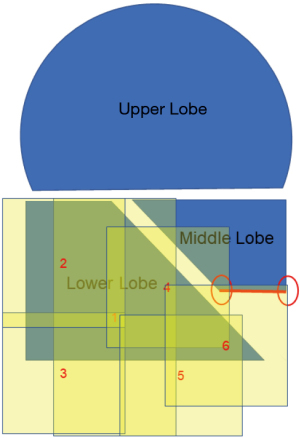
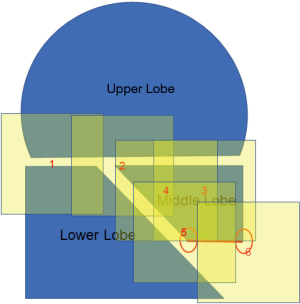
It is physically impossible to resect or ligate all lung cysts. Ruptured lung cysts or relatively large protruding lung cysts were cauterized by soft coagulation followed by application of fibrin glue and use of ENDOLOOP® Ligature made with PDS® II (ETHICON; Raritan, NJ, USA). The chest cavity was then flushed by sterile distilled water, and a water test was performed to confirm absence of a pulmonary fistula. U-VATS TPC was then performed. ORC mesh is easier to use if priority is given to the mediastinal or diaphragmatic side, which is difficult to cover. With approximately 50% inflation of the lung, TPC with sheets of ORC mesh encompassed the entire surface of the lung. In detail, we covered the right upper and middle lobe (Figure 2 and Video 1), followed by the basal area of the lower lobe (Figure 3 and Video 2), and the remaining lateral and posterior mediastinal lung surface of the lower lobe (Figure 4 and Video 2). Finally, the interlobar lung surface was covered (Figure 5 and Video 3). The patient underwent repeated expansion and collapse of the right lung to perform additional coverage until areas that were not covered were completely covered (Video 3). Finally, the degrees of lung expansion and pleural coverage were confirmed. After hemostasis is confirmed, a thoracic drainage tube was inserted through the same incision into the pleural cavity and the tube was sutured in place.
Postoperative considerations and tasks
Because the postoperative draining fluid was large (approximately 500 mL/day for several days) and the fluid seemed muddy because of the ORC decomposition products, a thin drain should be avoided. Postoperative management for the thoracic drainage tube was performed in a routine manner as follows: (I) continuous low-pressure suction (−7 hPa); (II) continuous periodic milking of the thoracic drain; and (III) maintenance of the drain in place until the daily volume of drainage was less than 200 mL. According to the published data of TPC (1), Intraoperative Clavien-Dindo Grade 3 or higher complications were not observed, and delayed postoperative pulmonary fistula was observed in 2 of 52 patients (3.8%). There were no intrapleural infections. The chest drains were removed on postoperative day 7.7±4.6, and the patients were discharged on postoperative day 9.5±4.8. There were no postoperative recurrences of pneumothorax (1).
Tips and pearls
The described procedures can be performed by uniportal VATS, as shown in the video. In the aforementioned publication (1), 14±2.3 ORC mesh (4×8") sheets were used and 9.1±2.7 mL of fibrin glue were used per patient. To avoid problems with insufficient expansion of the right lung associated with ORC mesh, fibrin glue was finally applied, and thrombin only could be applied, if needed, in the middle of this procedure. Perioperative monitoring specifically related to the surgical procedure was not required.
Discussion
BHDS is a rare inherited autosomal dominant genodermatosis caused by a germline FLCN mutation (7,8). The primary clinical manifestations of BHDS include fibrofolliculomas and trichodiscomas of the skin, renal tumors, and multiple lung cysts (9). The lung cysts in BHDS are predominantly located in the middle-to-lower lung fields, adjacent to the mediastinum, diaphragm, and interlobar area (4,5). These cysts are characterized by thin walls, round-to-oval shape, varying sizes, and their tendency to abut the peripheral pulmonary vessels (10). The unique characteristics of the BHDS cysts pose challenges to the standard surgical treatment of pneumothoraces, which typically involves resection and/or ligation of bullae. Consequently, pneumothoraces in BHDS patients are prone to recurrence (11).
A recent surgical procedure known as 4-port VATS TPC has been reported to be effective in preventing the recurrence of postoperative pneumothoraces in patients with BHDS (1). This procedure involves covering the entire surface of the lungs with sheets of ORC, which reinforce the entire pleura by inducing pleural thickening (1,2). Initially, this procedure required 4-port VATS. However, with advances in U-VATS techniques and instruments, U-VATS TPC has become a feasible alternative. Our team has successfully treated several cases of secondary pneumothorax in patients with BHDS or LAM with the use of the U-VATS TPC procedure and ORC mesh (12,13).
Conclusions
In this article, we provided a detailed description of the technique for the U-VATS TPC procedure and ORC mesh as a treatment for secondary pneumothorax in individuals with BHDS and hereditary multiple pulmonary cysts. Based on our experience, we believe that this technique is both safe and effective for managing secondary pneumothorax in patients with hereditary multiple pulmonary cysts.
Limitations
The design of this study has limitations. Firstly, this project was a retrospective observational analysis and included a small population of patients with BHDS. Further study should be performed prospectively. Secondly, U-VATS TPC is a special treatment for rare diseases, and its cost and societal adoption and impact have not been studied.
Acknowledgments
We thank JAM Post Biomedical Communications (Seattle, Washington, USA) for their excellent English language editing service.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://vats.amegroups.com/article/view/10.21037/vats-23-48/rc
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-23-48/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-23-48/coif). T.M. serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from August 2023 to July 2025. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Our local Ethical Review Committee determined that certification by the Ethical Committee and consent for publication from the patient were not necessary, because this paper contained a case report and a description of a surgical procedure that did not identify personal information.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mizobuchi T, Kurihara M, Ebana H, et al. A total pleural covering of absorbable cellulose mesh prevents pneumothorax recurrence in patients with Birt-Hogg-Dubé syndrome. Orphanet J Rare Dis 2018;13:78. [Crossref] [PubMed]
- Lee S, Kim HR, Cho S, et al. Staple line coverage after bullectomy for primary spontaneous pneumothorax: a randomized trial. Ann Thorac Surg 2014;98:2005-11. [Crossref] [PubMed]
- Ebana H, Hayashi T, Mitani K, et al. Oxidized regenerated cellulose induces pleural thickening in patients with pneumothorax: possible involvement of the mesothelial-mesenchymal transition. Surg Today 2018;48:462-72. [Crossref] [PubMed]
- Tobino K, Gunji Y, Kurihara M, et al. Characteristics of pulmonary cysts in Birt-Hogg-Dubé syndrome: thin-section CT findings of the chest in 12 patients. Eur J Radiol 2011;77:403-9. [Crossref] [PubMed]
- Tobino K, Hirai T, Johkoh T, et al. Differentiation between Birt-Hogg-Dubé syndrome and lymphangioleiomyomatosis: quantitative analysis of pulmonary cysts on computed tomography of the chest in 66 females. Eur J Radiol 2012;81:1340-6. [Crossref] [PubMed]
- Menko FH, van Steensel MA, Giraud S, et al. Birt-Hogg-Dubé syndrome: diagnosis and management. Lancet Oncol 2009;10:1199-206. [Crossref] [PubMed]
- Hornstein OP, Knickenberg M. Perifollicular fibromatosis cutis with polyps of the colon--a cutaneo-intestinal syndrome sui generis. Arch Dermatol Res 1975;253:161-75. [Crossref] [PubMed]
- Birt AR, Hogg GR, Dubé WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol 1977;113:1674-7. [Crossref] [PubMed]
- Zbar B, Alvord WG, Glenn G, et al. Risk of renal and colonic neoplasms and spontaneous pneumothorax in the Birt-Hogg-Dubé syndrome. Cancer Epidemiol Biomarkers Prev 2002;11:393-400. [PubMed]
- Kumasaka T, Hayashi T, Mitani K, et al. Characterization of pulmonary cysts in Birt-Hogg-Dubé syndrome: histopathological and morphometric analysis of 229 pulmonary cysts from 50 unrelated patients. Histopathology 2014;65:100-10. [Crossref] [PubMed]
- Gupta N, Kopras EJ, Henske EP, et al. Spontaneous Pneumothoraces in Patients with Birt-Hogg-Dubé Syndrome. Ann Am Thorac Soc 2017;14:706-13. [Crossref] [PubMed]
- Mizutani M, Mizobuchi T, Nagato K, et al. Uniportal video-assisted thoracic total pleural covering for a refractory pneumothorax in a patient with Birt-Hogg-Dubé syndrome: a case report. Video-assist Thorac Surg 2020;5:30. [Crossref]
- Oshima K, Mizobuchi T, Nagato K, Ishibashi F, Sugano I, Kumasaka T. Uniportal video-assisted thoracic total pleural covering for refractory pneumothorax in a patient with lymphangioleiomyomatosis: a case report. Video-assist Thorac Surg 2020;5:10. [Crossref]
Cite this article as: Mizobuchi T, Nagato K, Ito Y, Sobue A, Nakagawa A, Yagyu T. Uniportal video-assisted thoracic surgery for the creation of a total pleural covering for patients with Birt-Hogg-Dubé syndrome and secondary pneumothorax associated with hereditary multiple pulmonary cysts: surgical technique. Video-assist Thorac Surg 2023;8:46.


