
From video-assisted mediastinoscopy (VAM) to video-assisted mediastinal lymphadenectomy (VAMLA) in a low- and middle-income country: surgical technique
Highlight box
Surgical highlights
• Bimanual dissection during video-assisted mediastinoscopy (VAM), increases the possibility of performing a video-assisted mediastinoscopy lymphadenectomy (VAMLA) instead of lymph node biopsies or sampling.
What is conventional and what is novel/modified?
• When a video-mediastinoscope is not available, a traditional mediastinoscope with a 5 mm lens attached to the video camera can be used to facilitate the bimanual dissection.
• When a traditional mediastinoscope is used, an assistant should handle the video camera and hold the mediastinoscope so that the surgeon can operate with both hands.
• Bimanual dissection is more difficult when we use a conventional mediastinoscope because the lens occupies part of the working space.
• Differences between VAM to VAMLA are related to the difficulty of performing in-bloc resection.
What is the implication, and what should change now?
• VAMLA is a feasible procedure, rarely performed in low-and middle-income countries, improving and increasing its use could lead to better pathological staging of non-small cell lung cancer.
Introduction
Mediastinoscopy was introduced by Carlens in 1959 and excluding the underutilized extended cervical mediastinoscopy (ECM), this technique was hardly modified until 1992 when Linder and Dahan developed a two bladed video-mediastinoscope, with the Wolf company that had the possibility of bimanual preparation (1). Bimanual dissection increased the possibility of performing a mediastinal lymphadenectomy instead of lymph nodes biopsies (2). The specificity of traditional mediastinoscopy is almost 100% but its sensitivity depends on the amount of lymph node tissue resected and the number of stations biopsied ranging from 38% to 92% (3). Since 1999, Hürtgen et al. developed and described the use of the video-assisted mediastinal lymphadenectomy (VAMLA) to improve sensitivity (1), which has been reported as good as 93.8–97% (3-5).
Currently, health-care differences between high-income countries (HIC) and low- and middle-income countries (LMIC) are clear. The presence of obstacles to access health systems generates delays in care and worse results are frequent findings in these latter populations (6). Additionally, the absence of technologies that are routinely used in HIC marks a huge gap between these countries and LMIC. A video-mediastinoscope is one of them. In Colombia, it is only available in ten hospitals, all of them located in four major cities, only one of the three institutions where the authors work has video-mediastinoscope and although it is well known that mediastinal lymphadenectomy occupies a place as important as the pulmonary resection in the management of patients with lung cancer, VAMLA in not a very popular procedure.
Systematic lymphadenectomy by any approach is useful since the factor N is the most difficult and important in the tumor-node-metastasis (TNM) staging, due to its prognostic and therapeutic implications. In very few situations, lung resection procedures alone are considered the standard of care in the management of the most lethal neoplastic pathology in the world (1). Therefore, the International Association for the Study of Lung Cancer (IASLC) has included within the definition of complete resection a systematic lymphadenectomy that could be complete systematic lymphadenectomy or selective systematic lymphadenectomy that includes lobe-specific systematic nodal dissection, in addition to tumor-free margins in lung resection (7). In the past, describing that a complete extraction of the paratracheal, subcarinal, hilar and paraesophageal lymph node stations could be performed through the neck could be considered close to insanity. But, it is a reality that through a small incision in the neck a complete resection of these lymph node stations could be accomplished. The advantage of VAMLA is that it provides the most adequate preoperative diagnosis of factor N, as well as a more complete lymphadenectomy for the surgical treatment of non-small cell lung cancer (NSCLC).
The objective of this article is to show how to perform lymphadenectomy both with and without a video-mediastinoscope, how we have been improving our technique in a developing country where we do not always have a video-mediastinoscope, and thus, give tools to those who start in VAMLA to improve and get the most out of it.
Evaluation of the mediastinal lymph nodes in NSCLC is important as factor N has prognostic and therapeutic implications. This evaluation begins with images, computed tomography (CT) and positron emission tomography-computed tomography (PET-CT), but they are not always enough. Histological assessment of the mediastinum is indicated when the probability of N2 is greater than 10%, as in the presence of adenopathies on imaging or when the tumor is central or its size is 3 cm or more (8,9). This evaluation could be done by endobronchial ultrasound (EBUS), video-assisted mediastinoscopy (VAM), or VAMLA.
In cases where EBUS is available, after CT and PET-CT, it is used in the initial assessment of the mediastinal lymph nodes with an indication for histological assessment of the mediastinum. Thus, if the patient undergoes neoadjuvant therapy, restaging is performed with VAMLA; and when the EBUS is negative or not available, we confirm the absence of N2 with VAM or VAMLA. However, we prefer to do VAMLA to avoid re-mediastinoscopy, mainly in patients who are candidates for neoadjuvant therapy.
These are the main indications for VAMLA in our practice, and the contraindications are not absolute, but if the patient has limited neck extension or has had mediastinoscopy or radiation to the mediastinum, the contraindication is relative. We present this article in accordance with the SUPER reporting checklist (available at https://vats.amegroups.com/article/view/10.21037/vats-23-4/rc).
Surgical technique
The study did not involve human experimentation and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was not required as the study posed minimal risks. Nonetheless, our hospitals possess a written informed consent signed by patients, granting permission to use their anonymized videos or photographs for educational and scientific purposes.
In this minimally invasive technique, the incision is performed one fingerbreadth above the suprasternal notch, its size should be the same as the diameter of the blade of the video-mediastinoscope (Figure 1). The pretracheal space is prepared in the same way as in traditional mediastinoscopy with blunt dissection between the pretracheal fascia and the trachea to the carina; this is performed under general anesthesia with a single-lumen tube, maintaining mean arterial pressure around 70 mmHg and monitoring the oxygen saturation in the right hand, since it could alert to compression of the brachiocephalic arterial trunk.
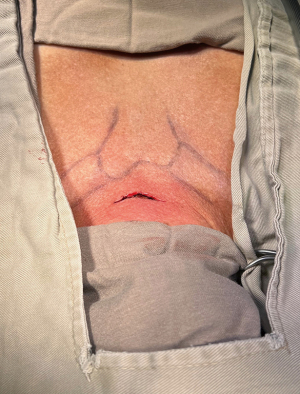
An alternative when a video-mediastinoscope is not available, is to use a traditional mediastinoscope with a 30-degree 5 mm lens connected to the video camera to perform this procedure, although with greater difficulty. In that case, it is necessary for an assistant to handle the video camera and sometimes hold the mediastinoscope so that the surgeon can operate with both hands simultaneously.
The first step is the introduction of the closed video-mediastinoscope in the pretracheal space. It is not necessary to use the holding device for fixation to the operation table because when the blades are opened, the video-mediastinoscope is fixed enough to maintain it in place. Blunt dissection with the aspirator tube and with advanced energy [ultrasonic system Harmonic® scalpel, Ethicon Endo-Surgery, Inc. (Cincinnati, Ohio, USA) or continuous bipolar energy LigaSureTM Maryland Jaw Open Sealer/Divider 5 mm–23 cm or LigaSureTM Maryland Jaw Thoracic Sealer/Divider 5 mm–30 cm, Covidien (Minneapolis, Minnesota, USA)], is made in order to identify the main bronchi, the carina, the right pulmonary artery and the left recurrent nerve (Video 1).
The introduction of the aspirator simultaneously with the advanced energy device is more difficult when we use a conventional mediastinoscope because the lens occupies part of the working space.
Advanced energy devices are more expensive; however, they are safer than monopolar devices during dissection within the mediastinum and are always available at our institutions, but with reuse policies. Additionally, when using ultrasonic energy, special care must be taken with the active blade because it could cause severe burns.
When the tissue located in the subcarinal region is separated from the right pulmonary artery, the resection is started using advanced energy to separate the specimen from the medial part of the left main bronchus, the bifurcation, and the right main bronchus, pushing the nodes caudally and sealing some small vessels. Then the lymph nodes and the fat are grasped and gently pulled to the proximal trachea making countertraction with the tip of the aspirator tube. After resecting this 7 station lymph nodes, the esophagus is exposed between the two main bronchi (Video 2).
The same sequence should be performed for station 7 dissection when using the traditional mediastinoscope and when we are performing VAM (Video 3).
The paraesophageal fat and lymph nodes (station 8) are dissected with the aspirator tube to the inferior pulmonary vein in both sides and it is the same dissection in VAM as in VAMLA. At this time, it is useful to check the hemostasis and leave some gauze pads in the subcarinal space.
The 4L and 10L stations can be dissected in bloc up and down of the tracheobronchial angle, respectively; several lymph nodes become visible next to the recurrent nerve. Special care must be taken when performing blunt dissection between the lymph nodes and the recurrent nerve in order to avoid nerve traction or injury. For this reason, the energy here should be avoided or used cautiously (Video 4). Occasionally, in the 2L station it could be difficult to find lymph nodes.
In the right-side, lymph nodes are located more anteriorly and the main bronchus can be easily exposed towards the upper lobe bronchus origin. Here, it is important to separate the nodes from the right pulmonary artery. The 10R, 4R and 2R stations are dissected from the tracheobronchial tree and the lymph nodes are pushed caudally to the left to expose the pleura, the azygos vein and superior vena cava; sometimes small venous vessels must be sealed in this dissection. 2R and 4R nodes can be resected in bloc to the azygos vein by cranial traction to the left once separated from the pleura and vena cava (Video 5).
Below the azygos vein, the 10R station is dissected in the same way as 4R station and resected by left-cranial traction (Video 6). After this dissection, is possible to see the upper lobe bronchus origin and the secondary carina, where in right-sided tumors, 11R station can be dissected.
In this paratracheal stations major differences exist between VAM and VAMLA, related to the difficulty of performing in-bloc resection that includes fatty tissue around lymph nodes.
Finally, hemostasis is checked and, if necessary, lavage with a solution of 1 mL of epinephrine 1 mg/mL in 250 mL of saline solution. This solution can also be used during dissection, when oozing blood obscures the field. Unfortunately, we do not have information registered about the time of the procedures performed.
The evaluation of intraoperative and postoperative surgical results is related to the absence of complications. Furthermore, it is based on complete resection of lymph nodes and surrounding fatty tissue from at least stations 2R, 2L, 4R, 4L, and 7 correlated with the histopathologic report.
Comments
Several advantages of VAMLA have been described: it is the option with the highest staging accuracy (10); it is the best option for pre-resectional lymphadenectomy, facilitating subsequent anatomical lung dissection (10,11); left paratracheal nodes can be completely resected specially in cases of N1 disease on the left side, or in patients with bilateral synchronous lung cancer (11) and because a complete lymphadenectomy was performed, it could prevent re-mediastinoscopies afterwards neoadjuvant treatment or avoid futile surgeries in high-risk patients (10).
The learning curve and its relationship with morbidity of VAMLA were recently analyzed by Daemen et al. (12) in a study performed in a single institution in the Netherlands, that included 236 VAMLAs for staging NSCLC by four surgeons performing their first VAMLA and followed for 7 years. They concluded that performing a procedure more frequently, every 13 days, allowed two of these surgeons to overcome the cut-off point associated with fewer complications in the learning curve, after 16–17 procedures. Complication rates of these surgeons decreased from 19–18% to 3–5%. Additionally, false-negative rate observed was 3% and there was no difference between the surgeons during the learning processes. Even in low volume services as in our country, the learning curve is shorter than the learning curve for EBUS (13), the alternative method to assess the mediastinal lymph nodes, especially in surgeons who had experience in conventional mediastinoscopy. If the video-mediastinoscope is scarce in our country, the EBUS is even more; furthermore, pulmonologists or thoracic surgeons are beginning their learning curve. The costs of the two procedures in our country probably are similar, because the EBUS transbronchial needle aspiration (TBNA) is always performed under general anesthesia. Even so, EBUS is safer than VAM or VAMLA (14). We are thoracic surgeons who have between 10 and 30 years of experience performing conventional mediastinoscopy; however, we began our training in VAMLA by watching videos and live procedures for at least 6 months; after that, we performed two VAMLAs in the company of an experienced thoracic surgeon, that has performed about 200 VAMLAs, and then we began to practice this with more and more confidence and now we can do this safely and confidently. Surgery is a collaborative effort involving a team; however, in our country it is not common to have the same team of anesthesiologists, operating room nurses, circulating nurses and assistants. The ideal situation is to have the same trained team in every surgery; but when this does not happen, in the preoperative period we teach at least the new members of the team the specifications of the equipment and the procedure.
If the dissection is guided by anatomical landmarks the procedure is quite safe and the number of lymph nodes obtained was higher than in open or video-assisted thoracic surgery lymphadenectomy (4). The advantages of VAMLA are mainly the improvement of visibility related to optics connected to a video system. It improves the teaching process, gives greater confidence to the surgeon, allows reaching stations that are not usually explored with the traditional mediastinoscope and facilitates performing more complex procedures, due to the possibility of using both hands (15). Assessment of the lymph nodes in the pre-resection period has clear indications that include: central tumors, >3 cm or N1 suspected by PET-CT or CT. In these cases, it offers a more precise staging, favoring the administration of more appropriate treatments.
Morbidity is similar to mediastinoscopy with about 4.6% (2.6–5.3%), and better sensitivity (93.8%), specificity (100%) and false negative rate 0.9% (4,15). The sensitivity, as in all the invasive mediastinal staging procedures, depends on the number of lymph nodes and stations evaluated and in VAMLA, due to the better vision and ease for the surgeon, an adequate number of nodes and stations can be evaluated more frequently. Left recurrent laryngeal paralysis is the most frequent complication in the different series (1.8%) (15), but paralysis on the right side is also possible. Special care must be taken to identify the left recurrent laryngeal nerve and avoiding the use of energy, mainly in the dissection of stations 2L and 4L which is not usually done in bloc. Although bleeding is not the most common complication (venous injury 1.38%) (16), it is the most feared. Lesions are usually of the azygos vein, right pulmonary artery, and, less frequently, the superior vena cava. As they are mainly low-pressure vessels, in the presence of small lesions, bleeding can be controlled initially with compression with gauze and, if possible, use hemo-clips or local hemostatic agents; however, if this is not sufficient, it should be converted to a sternotomy for adequate bleeding control. Additionally, mediastinitis has been also described; however, in our experience we have not seen this complication (15,17). To avoid complications, some landmarks should be clearly identified and dissected: right pulmonary artery before dissecting station 7 and 8; azygos vein and superior vena cava during dissection of stations 2R and 4R and recurrent laryngeal nerve before dissecting stations 2L and 4L. Sometimes it is not possible to complete VAMLA, due to the presence of calcified lymph nodes, intraoperative complications or technical problems with the video system of the mediastinoscope (15). In these situations, the possibility of not continuing with the procedure should be evaluated to avoid major complications.
We have performed VAMLA with the help of a 5 mm 30-degree lens introduced trough the traditional mediastinoscope; however, it is a more laborious procedure, requires an assistant and perhaps does not offer the same safety as when using a video-mediastinoscope. Nevertheless, some authors have mentioned that VAMLA is impossible to perform without the Linder and Dahan video-mediastinoscope (15), this could be the reason why this technique in LMIC is not performed as often as expected. We believe that it is safer and more comfortable to do it with the video-mediastinoscope; however, we also believe that when this device is not available, surgeons can perform more extensive resections like the ones we showed with the traditional mediastinoscope.
Transcervical Extended Mediastinal Lymphadenectomy (TEMLA) using sternal elevation and a video-mediastinoscope is more complex and currently is not used as a routine mediastinal staging procedure in lung cancer because it has been reported an increased number of complications as neurological or vascular lesions (left carotid artery injury and left laryngeal recurrent nerve palsy) (15,16). Meanwhile, Yendamuri and Demmy (18), described that using electromyographic monitoring (associated to avoidance of muscle relaxation), helped them to avoid recurrent laryngeal nerve injury in 18 cases performed. Additionally, they did not have TEMLA-related complications.
There is limited information about the use of VAMLA in LMIC. Sayar et al. (19), in a series carried out in Turkey in 2011, described that when a mediastinoscope is connected to a video system, brings some benefits like better dissection, less probability of bleeding, and facilitates surgical training. Later in 2013, in another series from Turkey, concluded that VAMLA was associated with better survival in NSCLC patients who underwent resection (20). Colombia, another LMIC, is the third most populous nation in all of Latin America, but there is one thoracic surgeon per 586,000 inhabitants, distributed in 18 major cities (21) and the video-mediastinoscope is only available in few hospitals located in four of those major cities. Despite this, thoracic surgeons perform this procedure with the traditional mediastinoscope with a 30-degree 5 mm lens connected to the video camera as described above. Some of the thoracic surgeons who perform VAMLA in our country have started to perform it after seeing this procedure performed in live surgeries during symposiums or after visits by thoracic surgeons or surgical groups to our country, which we consider have provided great benefits to our surgeons, similar to what was described in other LMIC in a recent meta-analysis (22). The authors are also convinced of the benefits that this procedure can bring, in terms of time, staging and surgical results, so we strongly recommend it, even when the video-mediastinoscope is not available.
Conclusions
VAMLA is a valuable preoperative staging procedure for NSCLC, but it is not widely adopted by thoracic surgeons in LMIC. Improving its performance may lead to optimal pathological mediastinal staging, particularly if EBUS is unavailable. This article outlines the VAMLA technique and its evolution from VAM, even in the absence of a video-mediastinoscope.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Carlos Galvez Munoz and Paula A. Ugalde Figueroa) for the series “Advanced Uniportal VATS” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://vats.amegroups.com/article/view/10.21037/vats-23-4/rc
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-23-4/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-23-4/coif). The series “Advanced Uniportal VATS” was commissioned by the editorial office without any funding or sponsorship. S.M. reports that she has received payments by participation in input meetings from MSD and also received payment for lectures from AstraZeneca and payment by participation in manuscript writing from Medtronic (Covidien). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study did not involve human experimentation and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was not required as the study posed minimal risks. Nonetheless, our hospitals possess a written informed consent signed by patients, granting permission to use their anonymized videos or photographs for educational and scientific purposes.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hürtgen M, Friedel G, Toomes H, et al. Radical video-assisted mediastinoscopic lymphadenectomy (VAMLA)--technique and first results. Eur J Cardiothorac Surg 2002;21:348-51. [Crossref] [PubMed]
- Leschber G, Holinka G, Linder A. Video-assisted mediastinoscopic lymphadenectomy (VAMLA)--a method for systematic mediastinal lymphnode dissection. Eur J Cardiothorac Surg 2003;24:192-5. [Crossref] [PubMed]
- Call S, Obiols C, Rami-Porta R. Present indications of surgical exploration of the mediastinum. J Thorac Dis 2018;10:S2601-10. [Crossref] [PubMed]
- Witte B, Hürtgen M. Video-assisted mediastinoscopic lymphadenectomy (VAMLA). J Thorac Oncol 2007;2:367-9. [Crossref] [PubMed]
- Call S, Obiols C, Rami-Porta R, et al. Video-Assisted Mediastinoscopic Lymphadenectomy for Staging Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:1326-33. [Crossref] [PubMed]
- Meara JG, Leather AJ, Hagander L, et al. Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Int J Obstet Anesth 2016;25:75-8. [Crossref] [PubMed]
- Rami-Porta R, Wittekind C, Goldstraw P. Complete Resection in Lung Cancer Surgery: From Definition to Validation and Beyond. J Thorac Oncol 2020;15:1815-8. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- De Leyn P, Dooms C, Kuzdzal J, et al. Preoperative mediastinal lymph node staging for non-small cell lung cancer: 2014 update of the 2007 ESTS guidelines. Transl Lung Cancer Res 2014;3:225-33. [PubMed]
- Hartert M, Tripsky J, Huertgen M. Video-assisted mediastinoscopic lymphadenectomy (VAMLA) for staging & treatment of non-small cell lung cancer (NSCLC). Mediastinum 2020;4:3. [Crossref] [PubMed]
- Leiro-Fernández V, Fernández-Villar A. Mediastinal staging for non-small cell lung cancer. Transl Lung Cancer Res 2021;10:496-505. [Crossref] [PubMed]
- Daemen JHT, van den Broek RAM, Lozekoot PWJ, et al. The learning curve of video-assisted mediastinoscopic lymphadenectomy for staging of non-small-cell lung carcinoma. Interact Cardiovasc Thorac Surg 2020;31:527-35. [Crossref] [PubMed]
- Lin CK, Lai CL, Chang LY, et al. Learning curve and advantages of endobronchial ultrasound-guided transbronchial needle aspiration as a first-line diagnostic and staging procedure. Thorac Cancer 2018;9:75-82. [Crossref] [PubMed]
- Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011;142:1393-400.e1. [Crossref] [PubMed]
- Witte B, Wolf M, Huertgen M, et al. Video-assisted mediastinoscopic surgery: clinical feasibility and accuracy of mediastinal lymph node staging. Ann Thorac Surg 2006;82:1821-7. [Crossref] [PubMed]
- Kużdżał J, Warmus J, Grochowski Z. Optimal mediastinal staging in non-small cell lung cancer: what is the role of TEMLA and VAMLA? Lung Cancer 2014;86:1-4. [Crossref] [PubMed]
- Lozekoot PWJ, Daemen JHT, van den Broek RR, et al. Surgical mediastinal lymph node staging for non-small-cell lung carcinoma. Transl Lung Cancer Res 2021;10:3645-58. [Crossref] [PubMed]
- Yendamuri S, Demmy TL. Is VAMLA/TEMLA the new standard of preresection staging of non-small cell lung cancer? J Thorac Cardiovasc Surg 2012;144:S14-7. [Crossref] [PubMed]
- Sayar A, Citak N, Metin M, et al. Comparison of video-assisted mediastinoscopy and video-assisted mediastinoscopic lymphadenectomy for lung cancer. Gen Thorac Cardiovasc Surg 2011;59:793-8. [Crossref] [PubMed]
- Turna A, Demirkaya A, Ozkul S, et al. Video-assisted mediastinoscopic lymphadenectomy is associated with better survival than mediastinoscopy in patients with resected non-small cell lung cancer. J Thorac Cardiovasc Surg 2013;146:774-80. [Crossref] [PubMed]
- Cardona AF, Mejía SA, Viola L, et al. Lung Cancer in Colombia. J Thorac Oncol 2022;17:953-60. [Crossref] [PubMed]
- Velin L, Lantz A, Ameh EA, et al. Systematic review of low-income and middle-income country perceptions of visiting surgical teams from high-income countries. BMJ Glob Health 2022;7:e008791. [Crossref] [PubMed]
Cite this article as: Martínez S, Peláez M, Beltrán R, Carvajal C. From video-assisted mediastinoscopy (VAM) to video-assisted mediastinal lymphadenectomy (VAMLA) in a low- and middle-income country: surgical technique. Video-assist Thorac Surg 2023;8:43.


