
Uniportal video-assisted thoracoscopic surgery Ivor Lewis esophagectomy: surgical technique
Highlight box
Surgical highlights
• This is the least invasive thoracic technique in Ivor Lewis esophagectomy. Intrathoracic side-to-side anastomosis technique described in the video is easy to perform and offers non-traumatized anastomotic edges.
What is conventional and what is novel/modified?
• Progression of minimally invasive techniques from conventional open techniques started with multiportal and reaches to the uniportal technique. Uniportal video-assisted thoracoscopic surgery (VATS) Ivor Lewis esophagectomy offers similar surgical and oncological results from a single incision. The circular anastomotic technique is the most frequently used one. We utilized side-to-side completely stapled technique in uniportal approach. This is an easy and fast technique to perform.
What is the implication, and what should change now?
• Uniportal VATS technique was described first in pulmonary resections and widely used nowadays. Feasibility of the technique in esophageal surgery was published in literature before. Perioperative advantages of uniportal rather than multiportal with same oncological outcomes make this technique stand out.
Introduction
There has been a transition from open surgery to multi-portal video-assisted thoracic surgery (VATS) because of the search for a minimally invasive technique, and it has now become a standard surgical technique for esophageal pathologies. It is known that the multi-portal technique provides less pulmonary complications, bleeding and shorter hospital stays compared to the open technique (1). In the search for the least invasive technique after multi-portal minimally invasive esophagectomy (MIE), the uniportal VATS technique was developed, mainly inspired from the experience in lung resections (2). Uniportal VATS technique, which is also widely applied today, is at least comparable in pain and morbidity to multi-portal VATS in patients with lung resection (3,4). There is limited data comparing the uniportal and multi-portal technique in esophageal surgery, but it is expected to yield similar results. Considering the course of the esophagus in the posterior mediastinum, robot assisted minimally invasive esophagectomy (RAMIE) provides a more flexible range of motion in a narrower area compared to VATS techniques. However, when factors such as the difficulty of accessing the robot in most centers and the high costs are considered, the least invasive VATS techniques remain up-to-date and feasible.
We shared this technique in 2017 and updated in 2022 (2,5). Some technical manipulation difficulties can be seen in uniportal VATS. To minimize these difficulties, technical details and instrumentation details will be explained in this article. We present this article in accordance with the SUPER reporting checklist (available at https://vats.amegroups.com/article/view/10.21037/vats-22-53/rc).
Preoperative preparations and requirements
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Council of Marmara University Faculty of Medicine (No. 09.2021.485; 6 December 2021) and informed written consent was obtained from all individual participants.
Preoperative preparation and patient selection for uniportal VATS esophagectomy are not different from multiportal VATS and open techniques. Routine laboratory tests, pulmonary and cardiac evaluations of all patients were performed. Thorax and upper abdominal computed tomography (CT) and positron emission tomography-computed tomography (PET-CT) are performed before surgery if the patient had malignant pathology. In addition, endoscopic evaluation is performed in all patients.
Absolute contraindications for multiportal VATS were defined when the technique was first described for lung resections (6). These are challenging situations related with experience of the surgeon and the team, severe and vascular pleural adhesions, previous surgery or irradiation and tracheobronchial reconstructions. However, these reasons are not absolute contraindications but rather conversion reasons, as more difficult cases can be done with VATS in the current era.
The surgical team includes an experienced surgeon, assistant (resident or attending surgeon), anesthesiologist and a nurse with general thoracic surgery experience. Although there is not much change in the surgical team, the changes in the anesthesia team are managed through effective communication with a preoperative patient review meeting. Performing the surgery in the hospital, which has experience in esophageal surgery, in management of intraoperative complications and also a dedicated team for postoperative care will reduce possible morbidity and mortality.
Step-by-step description
Abdominal phase, three-portal laparoscopy without a liver retractor
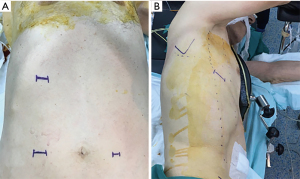
Three incisions are placed in the abdomen (Figure 1). Initially, a 12–15 mm laparoscopic port is placed 5 cm right lateral to the umbilicus and abdominal cavity is visualized. Second port (5 mm) is placed 5 cm left lateral to the umbilicus and the third port (10 mm) is placed in the middle of right costal arch.
Right periumbilical port is used for the camera. The right mid-costal port is initially used for the 10 mm Babcock clamp and the 5 mm port is used for the energy device. Dissection and division of the gastrohepatic ligament is the first step of the operation. The Babcock clamp is used both to lift the tissue and to retract the left lobe of the liver while left gastric region and hiatus are being explored (Figure 2A).
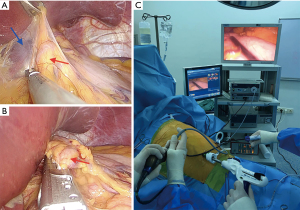
The celiac and left gastric lymph nodes are dissected with the surrounding adipose tissue to remain on the specimen for adequate lymphadenectomy and better visualization of the left hepatic, gastric and splenic artery. After preparation of left gastric vessels, camera position is changed to the mid-costal port. A 30–45 mm vascular stapler is inserted via the right periumbilical port while a 5 mm clamp lifts the left lobe of the liver through the left periumbilical port to expose left gastric area and gastric vessels is divided (Figure 2B). In patients with small caliber left gastric vessels, energy devices are used for division of those vessels.
Following this phase, omentum is lifted with the Babcock clamp and gastrocolic ligament is visualized. Babcock clamp is used with an open jaw maneuver without any grasping which allows lifting the stomach with one jaw of the clamp and retracting it to the right side.
Endoscopic staplers are used through the right periumbilical port. The tip of the stapler is curved to the medial side intracorporeally after the first stapler firing and typically 3–5 firings are performed for gastric conduit formation (Figure 2C).
Thoracic phase, uniportal VATS
Position and incision
All patients are evaluated for tracheobronchial system invasion with endoscopy and bronchoscopy on the operating table. Following double lumen intubation, the patients are placed in the left lateral decubitus position and tilted 30–45 degrees anteriorly. Especially in patients with narrow chest cavity and emphysematous patients, 45 degrees tilting provides better exposure. A 4-cm incision is made at the 6th intercostal space on the posterior axillary line (Figure 1). Using the incision at 6th intercostal space without crossing the posterior axillary line provides easy instrumentation and access to all parts of esophagus from hiatus to thoracic inlet.
Manipulations and positioning of instruments on incision
Regardless of the technique in esophagectomy, the procedures in the thorax are similar with small differences in open surgery and other minimally invasive techniques. The instrumentation and their placement in the incision determine the difficulty-ease or suitability of the techniques. In this section, we will describe the peculiarities of performing the procedure through a single incision and how it can be applied with the right instrumentation.
Division of the pulmonary ligament and opening of posterior mediastinal pleura
It is started by incising the inferior pulmonary ligament and the pleura over the esophagus by retracting the lower lobe antero-superiorly with forceps. Once the ligament is divided, inferior pulmonary vein and pericardium are our guides for anterior margin of resection. Then posterior mediastinal and paravertebral pleura over the esophagus were opened to azygos level. Meanwhile, the instruments are placed in the incision with forceps posterior to the incision, a camera in the middle, and an energy device anterior to the incision. In case of insufficient lung retraction, curved forceps with a peanut can push the lung and hilum anteriorly.
Deep dissection of esophagus anteriorly
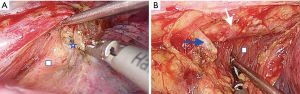
The pericardium provides a suitable avascular plane for dissection. By following this plane, the subcarinal lymph node can be released from the main bronchus and over the pericardium (Figure 3A). However, if there is a bulky lymph node or difficulty to create a dissection plan due to fibrosis on patients with neoadjuvant therapies, it can be removed separately.
Deep dissection of esophagus posteriorly after encircled with penrose drain
The esophagus is released from the paravertebral area by continuing the dissection until the aorta is seen at the fatty tissue under the hemiazygos vein. The small aortic branches of the esophagus should be carefully divided with an energy device and the dissection should be continued until contralateral vagus nerve is seen (Figure 3B). After the dissection is completed, the esophagus is encircled and lifted with a thick (2 cm wide) penrose drain. The penrose drain is then tautly placed and fixed with small clamp horizontally at the anterior side of the incision. After this stage, there is no need for retraction of the lung with forceps.
Dissection of subcarinal and left main bronchial lymph nodes
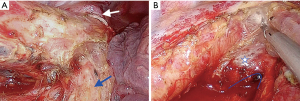
Dissection is continued in a deeper area over the left main bronchus and membranous trachea after the subcarinal lymph node is released from the pericardium and right main bronchus. The entire subcarinal area can be exposed by holding the penrose drain in the thorax with forceps very close to the esophagus. After sliding the penrose drain over the esophagus superiorly, lifting the drain and retracting it laterally and posteriorly exposed the whole subcarinal area (Figure 4). In this section, forceps for retraction of esophagus is located at the posterior part of the incision, camera at the middle and energy device at the anterior part of the incision.
Anastomosis
Intrathoracic anastomosis is the vital part of the MIE. In addition to the point to be considered during anastomosis, preparation before anastomosis is also important factor affecting anastomosis success. Both esophageal and gastric tissue quality should be evaluated before anastomosis and level should be decided accordingly. In patients with planned intrathoracic anastomosis, tissue perfusion should be preserved by avoiding extended dissection, especially in the cervical region. In patients receiving neoadjuvant radiation, care should be taken to ensure that the area of anastomosis is out of the radiation field. If any suspicion arises about the viability of esophageal wall, anastomosis should be planned at the neck. Another factor determining tissue quality is esophageal dilatation. In the area of esophageal dilatation secondary to both malignant and benign pathologies, deterioration of tissue perfusion can be observed as the wall thickness decreases. It is wise to perform anastomosis as high as possible close to the thoracic inlet. If intrathoracic anastomosis is not possible, neck anastomosis should be considered.
A side to side completely stapled anastomosis is preferred in our uniportal VATS Ivor Lewis esophagectomy technique (Video 1). The level of anastomosis is measured to allow a tensionless approximation. No. 1 silk suture is placed on the esophageal tip close to the stapler line for traction. Then, a small esophagostomy is opened at the esophageal end, and a nasogastric tube is advanced to the chest cavity which will serve as a guide to the stapler leg (Figure 5A). The gastric conduit is pulled out of the uniportal VATS incision in correct orientation and a small gastrostomy is opened at 5–6 cm away from tip of the conduit. The location of those incisions can be changed depending on the length of conduit and level of anastomosis. The thick leg of 60 mm tissue stapler is advanced in the gastrostomy (Figure 5B) and thin leg of stapler is placed in the esophageal opening taking the nasogastric tube as a guide. Posterior wall of the anastomosis is formed with a single firing (Figure 5C). This firing provides a 12 cm circumference for anastomosis. Stapler legs are usually 1–1.5 cm wide and if side closure is performed in the edge, it forms a circumference of 9 cm. This is almost equivalent to a circumference of a 28 mm circular stapler (8.8 cm). It is important that both edges of openings must be at the same level during stapler firing. After completion of posterior wall of the anastomosis, both ends are retracted towards the lateral chest wall and the anastomosis is completed with firing of one or two loads of 60 mm staplers (Figure 5D). During lateral wall firing care should be taken not to narrow the anastomosis, based on the calculation above.
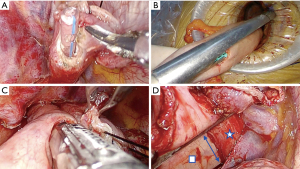
This is a no-touch anastomosis technique, the tips are not traumatized during manipulations. If the steps are followed carefully, it is an easy technique to apply and takes about 10–15 minutes. But there are a few points that need special attention. It is crucial to measure conduit and esophageal tip lengths correctly to perform tension free anastomosis. In this anastomosis technique, the point to be considered while creating the posterior wall is that the esophageal and gastric edges are aligned at the same line and uses the entire length of the 60 mm endostapler. If anastomosis is made without proper placement, the anastomotic diameter will be decreased, and anastomotic stenosis can be seen later. Another point for healthy anastomosis is that the ends of the esophagostomy and gastrostomy are sufficiently left between the stapler during the lateral wall formation. Anastomotic defects can be seen in cases where gastric and esophageal walls are misaligned and insufficiently placed between the legs of the stapler. In this technique, a long stapler line is formed on the lateral wall. Closure of this stapler line with pleura or fatty tissue may be beneficial in preventing fistula in possible healing problems. In our experience with two patients who were treated with curative chemoradiation, late fistula (2 months after surgery) occurred from the stapler line leading to lung abscess without pleural contamination.
Postoperative considerations and tasks
Reducing and preventing morbidity after esophageal surgery can be achieved with well-trained team and standardized approaches. Patients are followed in the ward or intensive care unit considering their comorbidities. In the early period of follow-up, effective analgesia protocols, early mobilization and pulmonary rehabilitation reduce the incidence of pneumonia. In addition, avoidance of hypotension is an important parameter to ensure perfusion of anastomosis. The chest tube is usually removed on the 4th day in patients with serous drainage who can tolerate clear liquids.
Oral nutrition initiation algorithm starts with water on the 3rd postoperative day. If there is no change in control chest X-ray and laboratory parameters, diet is advanced to puree and soft foods on day 5, respectively. Patients are advised to chew well and eat soft and puree diet until the second week after surgery.
Tips and pearls
- Uniportal VATS technique can be performed by following the described steps.
- Tilting the patient 45 degrees anteriorly provides a better exposure of posterior mediastinum.
- Side to side anastomosis can be performed quickly, easily and has comparable results with literature.
- The use of the 6th intercostal space provides a more suitable angle when creating the lateral wall during anastomosis.
- There is no study on the uniportal VATS esophagectomy learning curve, but the experience in the multiportal VATS technique is expected to shorten this period.
Discussion
Thoracic surgeons are currently turning to least invasive techniques. The uniportal VATS esophagectomy technique was defined by combining the experience of lung surgery with esophageal surgery from a 4 cm incision (2). Performing the same surgery from a single incision has become an important alternative to other minimally invasive and open techniques. This article shared the technical details of the technique.
Newly applied techniques should be comparable to standard esophagectomy techniques in terms of feasibility and oncological outcome. In the randomized controlled trial comparing the multi-portal and open esophagectomy techniques, significantly less pneumonia (P=0.005), shorter hospital stays (P=0.044) and less blood loss (P<0.001) were observed in the MIE group. There was no difference in postoperative leakage, 30-day mortality, R0 resection rate and number of total lymph node dissected as oncological parameters (7). Akhtar and colleagues reported similar results (8). According to 3-year follow-up results of TIME trial, no difference in disease free and overall survival was observed between MIE and open esophagectomy (9).
There are few publications in the literature on uniportal VATS esophagectomy. These were mainly for the purpose of esophageal release as part of a McKeown esophagectomy (10,11). In an article comparing multi-portal and uniportal MIE techniques, Lee and colleagues found no difference between total surgery time, length of hospital stays and total number of lymph nodes dissected. While pain scores were not different on the first postoperative day, the uniportal group showed significantly less pain scores on the 7th postoperative day (1.56 vs. 1.07, P=0.001) (12).
Feasibility of MIE was evaluated in prospective multicenter studies apart from retrospective papers. In the trial (ECOG-E2202) published by Luketich and colleagues, 95 of 104 patients evaluated with intent-to-treat model were completed with MIE (91%). Surgical outcomes were presented as 30-day mortality with 2.1%, anastomotic leak with 8.6% and 1-, 2-, 3-year survival with 80.5%, 68%, 68%, respectively. On the other hand, oncological outcomes were reported as R0 rate with 96% and the mean number of lymph nodes dissected as 19 (13). During the European Society of Thoracic Surgeons annual meeting in 2021, we presented an intent-to-treat population and recently published our results (14). Seventy-seven point five percent of 40 consecutive patients were completed uniportal and there was no conversion to open. Thirty- and 90-day mortality was 2.5% (n=1), 1- and 2-year survivals were 87% and 80%. R0 rate was 92.5% and the mean number of lymph node was 24 (15). Comparison of data with literature is presented in Table 1.
Table 1
| Author | Patients (n) | Technique | Location of anastomosis | Anastomotic leak, n (%) | Total time (minutes)* | Blood loss (mL)* | Complications, n (%) | 30-day mortality, n (%) | Lymph node dissected* | Conversion to open, n (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pulmonary | Cardiac | ||||||||||
| Fabbi et al. (16) | 36 | Multiportal | Intrathoracic | 2 (5.6) | 365 [240–480] | 100 [50–1,000] | 6 (16.7) | 4 (11.0) | NS | 24 [7–66] | – |
| Guo et al. (17) | 41 | Multiportal | Intrathoracic | 2 (4.8) | 268±38 | 207±74 | NS | NS | NS | 18.6±7.1 | 1 (2.4) |
| Biere et al. (7) | 59 | Multiportal | Neck and intrathoracic | 7 (12.0) | 329 [90–559] | 200 [20–1,200] | 7 (12.0) | NS | 1 (2.0) | 20 [3–44] | 8 (14.0) |
| Nachira et al. (11) | 12 | Uniportal | Neck | 2 (16.0) | – | – | 21 (6.7) | 4 (33.3) | NS | 10.4±3.9 | 0 |
| Lee et al. (18) | 16 | Uniportal | Neck and intrathoracic | 2 (12.0) | 608±93 | 288±361 | 0 | NS | NS | 30±14 | 1 (6.2) |
| White et al. (19) | 170 | Multiportal | Intrathoracic | 12 (7.1) | 391 [350–440] | 250 [50–2,500] | 8 (4.7) | NS | 1 (0.6) | 19 [14–24] | 8 (4.7) |
| Luketich et al. (20) | 1,033 | Multiportal | Neck and intrathoracic | 49 (5.0) | – | – | 85 (8.4) | 50 (4.9) | 17 (1.7) | 21 [15–29] | 45 (4.5) |
| Aslan et al. (14) | 40 | Uniportal | Neck and intrathoracic | 4 (11.7) | 160 [150–180] | 75 [25–150] | 3 (7.5) | 2 (5.0) | 1 (2.5) | 24±9.5 | 0 |
*, median values [interquartile range] or mean values ± standard deviation. NS, not stated.
Minimally invasive techniques are defined to provide the same surgical and oncological outcome with less morbidity and mortality. In addition, postoperative functional quality of life of patients is another important determinant. Early and late periods of Quality-of-Life data were presented by TIME trial. MIE offered better quality of life at 6-month and 1-year (9). It was also shown in the systematic review that the global health status started to improve after the 6th month in the MIE group correlated with TIME trial (21).
The leak determines the major morbidity and mortality of the surgery as the most feared complication. Although the anastomosis technique is determined according to the experience and familiarity of the surgeon, the circular stapler technique is the most frequently used one. Stapler shaft of circular stapler is not mobile and is straighter than fully curved linear endoscopic stapler. We frequently performed the anastomosis at high thoracic level. We needed an extra port in two patients because stapler angle was not suitable even in the fully curved position. A 28 mm circular stapler is used for a typical esophagogastric anastomosis and passage of the stapler through the intercostal space is very traumatic. Placement of pursestring suture is technically demanding and time-consuming in multi-portal VATS. Therefore, we don’t think use the circular stapler is feasible in the uniportal technique. We utilized side to side completely stapled anastomosis technique as a part of uniportal VATS Ivor Lewis esophagectomy. This is an easy and fast technique to perform. In patients with intrathoracic anastomosis, it takes a median of 12 minutes. Determining the appropriate anastomosis level by analyzing patient-related factors will reduce possible complications. Atraumatic, tension free, good mucosal approximated and well perfused tissue is crucial for success of anastomosis. Anastomosis should be avoided especially in the neoadjuvant radiated area. Leak rates in the MIE technique range from 4.8–16% (11,17). In the RAMIE Trial, it was reported as 12.2% and 11.3% (P=0.801) in the RAMIE and MIE groups, respectively (22). In our uniportal VATS Ivor Lewis esophagectomy technique, leak rate was 11.3% (n=3). Intraoperative prolonged hypotension and neoadjuvant radiation related unhealthy tissue are major factors related to these leaks.
Reaching the learning curve for MIE will make outcomes acceptable. Completion of learning curve for esophagectomy is expected to be shortened further with the widespread use of VATS and robotic techniques in other thoracic surgical pathologies. In the article, 5-year experience of the single surgeon RAMIE is shared, a statistically significant difference was found in the major complication rate, total operation time and hospital stay after 51 patients (23). Another article evaluating surgeons from four different centers where 646 patients were evaluated, the learning curve for anastomotic leak in patients with Ivor Lewis esophagectomy was reported as 119 patients and the leak rates decreased from 18.8% to 4.5% after learning curve (24). Another paper from single center experience published by White and colleagues. One hundred and seventy patients were included chronologically in four groups and no anastomotic leak was observed in the last group and this approached to be statistically significant (P=0.055) (19).
Conclusions
Uniportal VATS Ivor Lewis esophagectomy offers comparable outcomes to other MIE and open techniques from a single incision. It is seen as a feasible and safe technique when the technical details mentioned in this article are followed.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Carlos Galvez Munoz and Paula A. Ugalde Figueroa) for the series “Advanced Uniportal VATS” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://vats.amegroups.com/article/view/10.21037/vats-22-53/rc
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-22-53/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-22-53/coif). The series “Advanced Uniportal VATS” was commissioned by the editorial office without any funding or sponsorship. HB is a consultant with Johnson and Johnson, Medtronic and AstraZeneca and receives fees and honoraria, and he is also the Secretary General of European Society of Thoracic Surgeons (ESTS). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethical Council of Marmara University Faculty of Medicine (No. 09.2021.485; 6 December 2021) and informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Biere SS, Cuesta MA, van der Peet DL. Minimally invasive versus open esophagectomy for cancer: a systematic review and meta-analysis. Minerva Chir 2009;64:121-33. [PubMed]
- Batirel HF. Uniportal video-assisted thoracic surgery for esophageal cancer. J Vis Surg 2017;3:156. [Crossref] [PubMed]
- Jutley RS, Khalil MW, Rocco G. Uniportal vs standard three-port VATS technique for spontaneous pneumothorax: comparison of post-operative pain and residual paraesthesia. Eur J Cardiothorac Surg 2005;28:43-6. [Crossref] [PubMed]
- Reinersman JM, Passera E, Rocco G. Overview of uniportal video-assisted thoracic surgery (VATS): past and present. Ann Cardiothorac Surg 2016;5:112-7. [Crossref] [PubMed]
- Batirel H. Uniportal VATS Approach in Esophageal Cancer - How to Do It Update. Front Surg 2022;9:844796. [Crossref] [PubMed]
- Sihoe ADL. Are There Contraindications for Uniportal Video-Assisted Thoracic Surgery? Thorac Surg Clin 2017;27:373-80. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Akhtar NM, Chen D, Zhao Y, et al. Postoperative short-term outcomes of minimally invasive versus open esophagectomy for patients with esophageal cancer: An updated systematic review and meta-analysis. Thorac Cancer 2020;11:1465-75. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- Wang Q, Ping W, Cai Y, et al. Modified McKeown procedure with uniportal thoracoscope for upper or middle esophageal cancer: initial experience and preliminary results. J Thorac Dis 2019;11:4501-6. [Crossref] [PubMed]
- Nachira D, Meacci E, Mastromarino MG, et al. Initial experience with uniportal video-assisted thoracic surgery esophagectomy. J Thorac Dis 2018;10:S3686-95. [Crossref] [PubMed]
- Lee JM, Chen SC, Yang SM, et al. Comparison of single- and multi-incision minimally invasive esophagectomy (MIE) for treating esophageal cancer: a propensity-matched study. Surg Endosc 2017;31:2925-31. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Franchetti Y, et al. Minimally invasive esophagectomy: results of a prospective phase II multicenter trial-the eastern cooperative oncology group (E2202) study. Ann Surg 2015;261:702-7. [Crossref] [PubMed]
- Aslan S, Tiryaki GG, Pashayev J, et al. Uniportal video-assisted thoracoscopic surgery esophagectomy outcomes in 40 consecutive patients. Interdiscip Cardiovasc Thorac Surg 2023;36:ivad034. [Crossref] [PubMed]
- Huang GL, Yang L, Su M, et al. Vitamin D3 and beta-carotene deficiency is associated with risk of esophageal squamous cell carcinoma - results of a case-control study in China. Asian Pac J Cancer Prev 2014;15:819-23. [Crossref] [PubMed]
- Fabbi M, De Pascale S, Ascari F, et al. Side-to-side esophagogastric anastomosis for minimally invasive Ivor-Lewis esophagectomy: operative technique and short-term outcomes. Updates Surg 2021;73:1837-47. [Crossref] [PubMed]
- Guo W, Ma L, Zhang Y, et al. Totally minimally invasive Ivor-Lewis esophagectomy with single-utility incision video-assisted thoracoscopic surgery for treatment of mid-lower esophageal cancer. Dis Esophagus 2016;29:139-45. [Crossref] [PubMed]
- Lee JM, Yang SM, Yang PW, et al. Single-incision laparo-thoracoscopic minimally invasive oesophagectomy to treat oesophageal cancer†. Eur J Cardiothorac Surg 2016;49:i59-63. [PubMed]
- White A, Kucukak S, Lee DN, et al. Ivor Lewis minimally invasive esophagectomy for esophageal cancer: An excellent operation that improves with experience. J Thorac Cardiovasc Surg 2019;157:783-9. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Taioli E, Schwartz RM, Lieberman-Cribbin W, et al. Quality of Life after Open or Minimally Invasive Esophagectomy in Patients With Esophageal Cancer-A Systematic Review. Semin Thorac Cardiovasc Surg 2017;29:377-90. [Crossref] [PubMed]
- Yang Y, Li B, Yi J, et al. Robot-assisted Versus Conventional Minimally Invasive Esophagectomy for Resectable Esophageal Squamous Cell Carcinoma: Early Results of a Multicenter Randomized Controlled Trial: the RAMIE Trial. Ann Surg 2022;275:646-53. [Crossref] [PubMed]
- Han Y, Zhang Y, Zhang W, et al. Learning curve for robot-assisted Ivor Lewis esophagectomy. Dis Esophagus 2022;35:doab026. [Crossref] [PubMed]
- van Workum F, Stenstra MHBC, Berkelmans GHK, et al. Learning Curve and Associated Morbidity of Minimally Invasive Esophagectomy: A Retrospective Multicenter Study. Ann Surg 2019;269:88-94. [Crossref] [PubMed]
Cite this article as: Aslan S, Batirel H. Uniportal video-assisted thoracoscopic surgery Ivor Lewis esophagectomy: surgical technique. Video-assist Thorac Surg 2023;8:44.


