
Bleeding control during uniportal video-assisted thoracic surgery without conversion: evidence and technical aspects
Introduction
Since the first series of video-assisted thoracic surgery (VATS) lobectomies was published in 1992 (1), minimally invasive thoracic surgery (MITS) has widely spread worldwide not only due to the evidence regarding the advantages compared to traditional open approach (2-5), but also for an exciting shift in the specialty from a kind of old-fashioned approach towards a new age. Despite almost thirty years of evolution and after performance of every single challenging procedure through VATS, its practice within the thoracic surgery community is still far from desired, accounting only 35.2% of VATS procedures in the 2020 annual report from European Society of Thoracic Surgery (ESTS) database (6).
Some of the reasons for this low rate of VATS performance are the lack for systematization, oncological concerns and controversies, technical difficulties in bronchovascular dissection and fear of dealing with intraoperative complications, mainly vascular injuries with bleeding that constitutes the most frequent reason for emergency conversion to thoracotomy (7).
After first report of a lobectomy through a single incision in 2010 (8), interest in uniportal VATS grew motivated not only for a mild better profile in postoperative parameters such as shorter chest tube duration, shorter hospital stay and lower postoperative pain (9-11), but also for the concurrence of technological improvements and new initiatives for better and faster teaching the techniques by more media-conscious surgeons.
We aim to analyze the evidence regarding intraoperative bleeding during uniportal VATS procedures, and to describe different techniques for bleeding control without conversion.
Incidence of operative bleeding
Intraoperative bleeding is the most feared complication during VATS procedures, especially if occurs while dissecting the pulmonary vessels. Intraoperative bleeding during VATS is uncommon in experienced hands (less than 1%) (12,13) accounting for only 0.5–5.2% of the reasons for conversion in some of the biggest series (14). But the real incidence within the general practice in conventional centers is probably underestimated because successful attempts of managing through VATS are usually not indicated and reported in databases, and more severe and catastrophic situations are often not published. In large published series, the rate of massive bleeding due to vascular injury during VATS major resection ranges between 2.9–8.3% (7,15-20).
Pulmonary circulation is a low-pressure circuit, thus vessel walls are thinner than systemic vessel walls, but as it carries blood from the heart to the lungs for oxygenation and then back again to the heart, it takes very high-flow of blood throughout (14). These special features require that vascular dissection of pulmonary vessels be performed very gently, what is not easy when dealing with calcified or metastatic lymph nodes or tumors mainly in contact with branches of pulmonary artery (PA). If injured, especially in central branches of the PA, massive bleeding to manage through small incision can determine conversion to thoracotomy (79.5% of intraoperative bleedings converted to open thoracotomy in a series of 3,076 VATS major resections (18), more extensive resections than planned or even exsanguination with fatal consequences to the patient.
If specifically searching for uniportal VATS reports, there is an unexplainable lack of uniportal databases with only single-center (21,22) or scarce multicenter reports. A 4-year experience from a single center after adoption of uniportal VATS showed that after 60 procedures, the operative time decreased significantly, but almost 140 procedures were needed for mastering in this approach thus reflecting the difficulty of achieving proficiency in uniportal VATS (21). Previous experience in VATS has shown to decrease the rate of conversion to thoracotomy during the initial 50 cases of uniportal VATS (4% vs. 12%; P=0.018) but there is no specific data comparing the causes for such conversions (23). Some unicentric comparative studies have shown lower intraoperative blood loss during uniportal when compared to multiportal VATS, but without significant differences in conversion to thoracotomy (P=0.180) (24). Two different meta-analysis published in 2020 (25) and 2021 (26) comparing uniportal and multiportal VATS found no statistically significant differences in terms of blood loss (P=0.18 and 0.12, respectively) and conversion rate (P=0.14 and 0.68) but there is no specific data regarding intraoperative vascular injuries (Table 1).
Table 1
| Category | n | OR/WMD (95% CI) | P | Heterogeneity (I2) | Heterogeneity (p) |
|---|---|---|---|---|---|
| 2020 | |||||
| Conversions | 13 | 1.27 (0.83, 1.94) | 0.14 | 13% | 0.32 |
| Blood loss | 14 | −0.14 (−0.35, 0.06) | 0.18 | 86% | <0.01 |
| 2021 | |||||
| Total complications | 35 | 0.76 (0.62, 0.93) | 0.008 | 4% | 0.41 |
| Conversions | 11 | 0.91 (0.56, 1.46) | 0.68 | 0% | 0.49 |
| Blood loss | 14 | −11.64 (−26.34, 3.06) | 0.12 | 97% | <0.01 |
VATS, video-assisted thoracic surgery; OR, odds ratio; WMD, weighted mean difference; CI, confidence interval.
Most published series of uniportal VATS have been done probably more as an attempt for vindicating safety and feasibility for uniportal VATS against mistrust by some thoracic surgeons, than really as a rigorous and sincere exercise for collecting multicentre experience in high-volume series or databases. Beside the past debate of uniportal compared to multiportal VATS, there is a need for exhaustive analysis of uniportal VATS results after 12 years of adoption for anatomical resections worldwide in order to support strong evidence and shed a light for further research. Information regarding specific intraoperative complications and how they are managed should be described.
In a 2-center retrospective cohort of 442 uniportal VATS anatomical resections, authors reported 4.5% unexpected major vascular bleeding (vessel injury requiring immediate compression due to excessive blood loss, unstable vital signs, or unavailable to sponge management within 1–2 minutes). Only 0.9% of total cases were bleeding-related conversions to thoracotomy, and 0.45% were bleeding-related conversions to two port approach (27).
Causes of vascular injury
Vascular injury from pulmonary vessels is the most common cause of intraoperative bleeding while performing uniportal VATS procedures and also the main safety concern due to the specific features above mentioned (28).
There are several potential causes for vascular injury from pulmonary vessels while performing anatomical resections that can be summarized as follows:
- Surgeon’s negligence (
Video 1); - Accidental injury secondary to electrocautery or energy devices (
Video 2); - Malfunction of vascular endostaplers, accidental injuries while introducing vascular endostaplers or endoclips (
Video 3), or displacement of vascular endoclips; - Dissection-related vascular injury secondary to fibrosis, calcified or metastatic lymph nodes, or tumor adherence to PA (
Video 4); - Vascular tears secondary to traction (
Video 5), blunt dissection of the vessels ( Video 6) or dissection of other structures (fissure, bronchus, etc.).
All these potential sources of vascular injury are even more frequent during the learning curve (21,24), and acute management of a crisis situation appears more challenging for traditional maneuvers of bleeding control through a unique incision. Preoperative assessment of the specific risk for bleeding and preventive measures as we will discuss later, are extraordinary valuable while overcoming this learning period until mastering in terms of patient’s safety but also surgeon’s own health.
On the other hand, induction treatment for non-small cell lung cancer (NSCLC) induces necrosis and fibrosis due to tumor response to treatment, what makes bronchovascular dissection more difficult and challenging (29). Traditionally indicated for locally advanced clinical stage IIIA, there are many clinical trials ongoing including induction treatment for earlier stages (cIb–IIIA), and many of them include immunotherapy as a single or combined treatment which has shown not negligible pathological responses (30-32). The conjunction of more extended minimally invasive approach worldwide and more patients operated after induction treatment with new agents creates a different scenario that has to be carefully addressed by thoracic surgeons in order to preserve patient’s safety throughout the whole procedure.
Classification of intraoperative bleeding
There are no specific classifications of severity of intraoperative bleeding (33), but depending on the location, the blood flow and the possibility of intralobar or hilar PA control, it can be divided into uncommonly threatening (mild), potentially threatening (moderate), and immediately threatening for patient’s life (severe).
- Mild bleeding: usually occurs in stapled vascular stumps, in small segmental or subsegmental branches, or in tears in PA during pulmonary dissection (
Video 7). This mild bleeding commonly stops applying compression with a sponge stick or suction device, and usually does not compromise patient’s life. Application of sponge stick after immersion into hot saline can quickly activate coagulation and create a thrombus. Hemostatic products and sealants can also be applied for ensuring hemostasis before continuing the dissection. Time and compression are usually enough for controlling these mild bleedings. After hemostasis, surgical maneuvers and dissection should be carried gently in order to avoid re-bleeding. In stapled stump bleedings and after compression to create a coagulum, primary suture with 5/0 non-absorbable monofilament solves definitely the problem. Electrocautery in vascular stumps can be reckless for the possibility of increasing the vascular tear. For small branches, the use of endoscopic metallic or polymeric clips, or primary ligation with silk suture is usually enough for definitive solution. - Moderate bleeding: small tears in proximal PA or hilar branches (superior trunk on right side; A3, A1+2a+b, A1+2c on the left side) or more significant injury in PA in the fissure (
Video 8), leads the surgeon to a more challenging situation. Moderate bleeding potentially threats patient’s life if no quick control is achieved. Immediate compression with effective control of the bleeding is essential in order to avoid emergent conversion to thoracotomy. Then, sequential specific techniques for definitive repair will be discussed later. - Severe bleeding: significant injuries in proximal PA or hilar branches (
Video 9), or massive injuries in PA in the fissure really expose the patient to a life-threatening situation. Immediate compression under direct view with effective control of the bleeding is mandatory, but emergent conversion to thoracotomy is usually recommended and multidisciplinary approach with nursing staff and anesthesiologist must be initiated. Specific techniques will be discussed later.
Prevention and preparation for the bleeding
There are two concepts that become extraordinary valuable when dealing with complex surgeries exposed to risk of bleeding such as pulmonary major resections: prevention and preparation. The best bleeding management is the one that can be avoided.
Prevention of the bleeding includes all educational and technological resources available for minimizing the chance of a vascular injury.
Educational: training in structured units with systematical learning in procedures of progressive levels of difficulty is necessary in order to acquire comprehensive training in the speciality. Exposing assistant trainees to emergent situations managed by experienced surgeons poses a safe environment for achievement of essential skills. Simulation activities with physical models [Fukuoka Trainer (34), Ethicon Stupnik VATS simulator], videothoracoscopic simulators (35) and virtual/augmented reality (36) offer additional tools for exposing surgeons to basic maneuvers and conventional pulmonary major resections. By contrast, there is no simulation scenario available nowadays that mimics a real emergent situation such as vascular injury, so we should encourage and cooperate with technological industries for this purpose. Visualization of videos through surgical websites (37-39) or channels is useful for teaching skills in order to deal with this specific complication. Practical courses on animal models offer a good chance for training in scenarios in-vivo proctored by experienced surgeons.
Technological: the improvement of surgical devices for safely dissection during thoracic surgery has been extraordinary in the last decade, with advanced energy devices (mainly advanced bipolar and ultrasonic energy devices) that enable the surgeon not only to blunt dissect, but even safely seal and divide vessels up to 7–8 mm diameter. Some of these advanced devices have been designed specifically for VATS procedures. Besides this, last generation endostaplers with new stapling design for reinforcing the vascular stumps, and curved blunt tip anvils decrease the possibility of vascular injury due to malfunctioning.
On the other hand, preparation for the bleeding includes specific techniques before and during the surgery that anticipate a potential vascular injury protecting the patient’s life.
Exhaustive visualization of computed tomography (CT) scan and three-dimensional (3D) models: preoperative work-up with detailed analysis of past history of infectious or inflammatory disease (tuberculosis, pneumoconiosis) (33) is necessary. Central tumors or those with N1 malignant lymph nodes in contact with PA, require detailed analysis of intravenous contrast-enhanced CT which provides information about potential invasion of PA or its branches (40). 3D models based on contrast-enhanced thin-section CT scan offer that information in more illustrative reconstructions (41-43) showing segmental branches of PA and anatomical variations (28).
Emergency tray: in cases where a potential vascular injury can be anticipated, setting up of an emergency tray with specific material for conversion to thoracotomy is essential. Shears for dividing the wound protector, Finochietto retractor for rib spread, vascular clamps for PA, Satinsky DeBakey clamps, 4/0 and 5/0 non-absorbable monofilament sutures, and two independent suction devices are useful tools for safely dealing with a significant bleeding (44).
Preoperative debriefing with nursing and anesthesiology staff: all the professionals involved in the operating room (OR) should discuss preoperatively cases where risk for bleeding is present in order to be ready for the procedure.
Experience in uniportal VATS: when dealing with this troublesome situation, experienced surgeons are worth it for successfully managing the emergency (45). The role of an experienced assistant is crucial in order to keep the thoracoscope inside the cavity for immediately compression, and if the blood stains the optic, assistant must quickly take the thoracoscope out for clearance and back again inside the cavity for achieving compression of the bleeding site.
Control of PA: if the surgeon predicts a moderate-high risk for vascular injury, initial proximal/distal control of ipsilateral PA with rubber vessel-loops or silk sutures is probably the most useful and simple tip for safely dealing with challenging cases.
- Control of proximal PA: proximal control of PA provides an extremely useful tool for dealing with intraoperative moderate-severe bleeding, reducing the incoming blood flow (Figure 1A). In order to avoid displacement of the loop should a proximal tear in PA is present or sleeve division is performed, is better to keep some bronchovascular tissue in the right side (Figure 1B) (Figure 1C), or even perform an intrapericardial control of PA (Figure 1D).
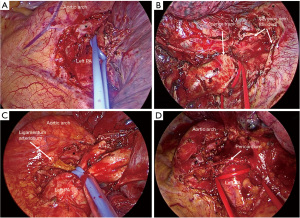 Figure 1 Pulmonary artery control. (A) Direct pulmonary artery control with a vessel-loop. (B) Right pulmonary artery control with remaining bronchoarterial tissue. (C) Left pulmonary artery control proximal to ligamentum arteriosum. (D) Intrapericardial left pulmonary artery control. *, vagus nerve. PA, pulmonary artery.
Figure 1 Pulmonary artery control. (A) Direct pulmonary artery control with a vessel-loop. (B) Right pulmonary artery control with remaining bronchoarterial tissue. (C) Left pulmonary artery control proximal to ligamentum arteriosum. (D) Intrapericardial left pulmonary artery control. *, vagus nerve. PA, pulmonary artery. - Control of PA in the fissure: distal control of PA decreases the backward flow facilitating vascular repair. For lower lobe difficult segmentectomies or lobectomies, getting control of PA in the fissure is a simple but effective method for safely attempting vascular dissection (Figure 2). In cases of potential vascular sleeve in the upper lobes, control of PA in the fissure is advisable for further setting of vascular tourniquets, and for that purpose, segmental control is an alternative (Figure 3).
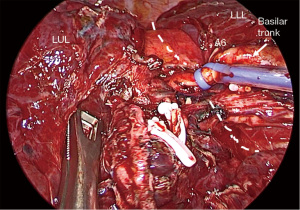 Figure 2 Control of pulmonary artery in the fissure. The dashed line indicates fissure location. LUL, left-upper lobe; LLL, left-lower lobe.
Figure 2 Control of pulmonary artery in the fissure. The dashed line indicates fissure location. LUL, left-upper lobe; LLL, left-lower lobe.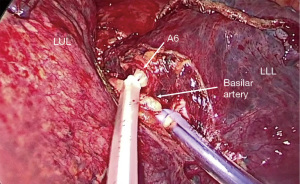 Figure 3 Segmental control of pulmonary artery in the fissure. LUL, left-upper lobe; LLL, left-lower lobe.
Figure 3 Segmental control of pulmonary artery in the fissure. LUL, left-upper lobe; LLL, left-lower lobe.- Control of pulmonary vein: in cases of blocked fissure due to central tumors or lymph nodes, in addition to proximal control of PA, clamping of inferior pulmonary vein protects from back-flow should a vascular sleeve or PA reconstruction is finally necessary.
- Tourniquet’s setting: controlling the PA with traditional clamps through a unique 3–5 cm incision is feasible but hinders the surgeon’s accessibility for handling dissection instruments and the video thoracoscope. Commercial intrathoracic tourniquets are available but can also be easily home made with a metallic hook inside a suction probe of about 10 cm length, where the vessel loop/silk is introduced. When is necessary, pulling the tourniquet to the incision and pushing the suction probe towards the hilum occludes the artery (Figure 4). Tourniquets are then introduced inside the pleural space so the incision is left clear from clamps and tourniquets. Intracavitary tourniquets are an outstanding tool for dissection in high-risk areas during major resections.
Techniques for bleeding control
There are several publications describing specific techniques for bleeding control through VATS or uniportal VATS, and some review papers summarizing the available methods (14,28,46,47). Here we describe and illustrate from initial to more complex maneuvers for control of vascular injury through uniportal VATS.
Direct compression
When bleeding occurs, immediate compression of the bleeding site is the first and mandatory measure, as an imitation of finger compression in open surgery (28,33). Sponge-stick, gauze-balls and suction are the most effective instruments, but any instrument that allows compression of the vessel against mediastinum or any fixed structure like bronchus can be useful (28) (Video 12). About 5 minutes compression is recommended, but after the first 1–2 minutes, a quick sight with slight decompression of the bleeding site provides information about the severity of the injury (33).
Side compression with suction provides not only mechanical decrease of blood flow through the arterial tear, but also clears up the field of blood bringing more brightness and calm to the situation. Curved tip suction is recommended in order to leave space for further techniques of definitive repair of the injury through a unique incision (33).
In the meanwhile of requesting for a compression instrument, direct compression of the bleeding site with the nearest lung parenchyma offers initial partial or entire control. If direct compression is not entirely effective, conversion to thoracotomy should not be delayed, but at least partial control is advisable because otherwise the high-flow through PA injuries will probably exsanguinate the patient before thoracotomy retractors can be completely opened.
Hemostatics, sealants, patches
Vascular injuries up to 6 mm can be managed safely with topic sealants (28). Regarding the difficulty for suturing through uniportal VATS, these agents are recommended as first-line measures. Their mechanisms of action are mainly: hemostasis, stopping bleeding and promoting coagulation; sealant, stopping leakage of blood in this case.
Hemostatics have different presentations: mechanical [porcine gelatin—Gelfoam® (Upjohn, Kalamazoo, MI, USA); bovine collagen—Ultrafoam® (Woburn, MA, USA); cellulose matrix—Surgicel® (West Somerville, NJ, USA); polysaccharide spheres—Arista® (Woburn, MA, USA)], active [thrombin-based—Thrombin® (West Somerville, NJ, USA)], liquids [FloSeal® (Round Lake, IL, USA); Surgiflo® (West Somerville, NJ, USA)] and fibrin sealants [Tissel® (Deerfield, IL, USA); TachoSil® (Linz, Austria)] (33).
Topic sealants are useful but require a dry field, which is difficult when a tear is made in the PA. They provide a thin layer covering the injury to seal the leakage of blood. They have liquid and pulverized presentations adapted for VATS application. Fibrin, albumin + glutaraldehyde and polietilenglicol are their main components [Tiseel® (Deerfield, IL, USA), Bioglue® (N.W.Kennesaw, GA, USA), Coseal® (Round Lake, IL, USA)].
Some patch products combine both features of activation of fibrin clot and sealant of the injury (Tachosil®) but its application through uniportal while keeping aspiration of blood from the vascular tear is not easy and requires skillful handling.
Endoclips and ligatures
For injuries in branches of the PA, if not located at the root itself, endoscopic metallic or polymer clips and sutures can be applied. Traditional metallic clips are useful in side tears of vessel branches if complete dissection of the vessel has not been previously done or it’s hindered by blood in the field and compression instruments (Video 13). Locking polymer clips [Hem-o-lok® (Morrisville, NC, USA); Click’aV®] offer excellent outcomes in terms of safety with angulating and rotating appliers for better visualization of distal end of the clip. Depending on the size of the vessel, use of proximal clip with distal sealing with advanced energy devices seems the best option for bleeding control while keeping distal specimen clear from foreign material that can be displaced with maneuvers or included in fissure or intersegmental planes of division (33).
Traditional silk ligatures can also be applied proximally to injuries in PA branches and tightened with a VATS knot pusher through the incision (Video 14).
Direct suture repair
If the bleeding is not controlled with the previous techniques, primary suture should be attempted for vascular repair. Before starting the suture, keep compression at the injured site as previously described and request for a 5/0 non-absorbable monofilament (Prolene®, GoreTex®), a needle-holder and suction for clearing up the field from blood, and also share with anesthesiologist and nursing staff next steps (33).
As primary suture through uniportal VATS is challenging, positioning of instruments is important for an easier technique of suturing (thoracoscope in the posterior aspect; suction and needle-holder in the anterior aspect). Experienced uniportal surgeons perform this direct suture while the bleeding is active, by using the suction to clear up the field as possible, and using needle-holder both for suturing and also grabbing the needle again (28) (Video 15).
Most of the techniques for primary repair can be categorized within the concept of “Suction Compressing Angiorrhaphy Technique” (SCAT) as described in 2013 depending on the size of the injury. In a large series of 414 major resections, 88% of the intraoperative bleedings due to vascular injury were successfully repaired with the SCAT technique (20).
Rotating suture angiorrhaphy (defects <5 mm): while performing side compression with the tip of the suction in one side of the defect, a first stitch is made in the other side. Then the tip of the suction is slightly moved to the opposite site, while a second stitch is made in the other side of the defect (28,48) (Video 16).
Clamping angiorrhaphy (defects >5 mm and <1/3 circumference): for bigger defects not involving more than 1/3 of the circumference of the vessel, atraumatic clamp like Allis forceps can be used for clamping the edges of the vascular wound, then removing the suction and afterwards performing the suture in the same manner as described before (20,33) (Figure 5). Try to avoid blind clamping of the vessel wound or the proximal artery because there is risk for increasing the injury and dissecting the artery proximally.

Blocking angiorrhaphy (defects >5 mm and >1/3 circumference): for larger defects exceeding 1/3 of the vessel circumference, proximal clamping of the vessel should be attempted (20,48). After initial suction compression and substitution for clamping the edges of the wound, a proximal dissection and circumferential control of the vessel is performed using a vessel-loop/tourniquet (49) (Figure 6), an endoscopic bulldog (48,50) or an atraumatic vascular clamp (a clamp is less advisable through uniportal unless an additional port is performed).

Conversion to thoracotomy
After unsuccessful initial attempts for vascular repair, or if the bleeding exceeds the ability for suction and compression, conversion to thoracotomy cannot be delayed. Some situations must be considered an indication for conversion (28):
- Large vessel injuries with massive bleeding and inadequate vision;
- Extension of initial injury after initial attempts for uniportal repair;
- Hemodynamic instability or severe hypovolemia;
- Insufficient experience of the team;
- Exhaustion of the surgeon during challenging long-lasting procedures.
We recommend keeping thoracoscopic view of the bleeding site for checking adequate compression and control in the meanwhile of conversion to thoracotomy, that has to be done in an expeditious manner. Division of the metallic ring of the surgical wound protector can be done with bone shears facilitating conversion to open approach. An adequate conversion tray with necessary instruments should be set up in high-risk procedures (Figure 7). An extension of the uniportal incision must be quickly performed, mainly posteriorly. Adequate hemostasia from chest wall should be delayed if the bleeding is not completely controlled under compression or the patient is hemodynamically unstable.

Strengths and limitations
This paper is a Clinical Practice Review, which summarizes both clinical and surgical issues regarding causes of vascular injury during uniportal VATS pulmonary resection, preventive measures and surgical considerations for dealing with intraoperative bleeding. It has been written under the light of experience of the author’s, combining this empiric information with current evidence available in scientific publications.
Humbleness of the authors combined with academic and educational interest are its main strengths. Its limitations are especially the low level of evidence available for its development, and the low degree of recommendation that can be addressed from it.
Conclusions
Intraoperative bleeding during uniportal VATS anatomical resections, despite uncommon in experienced hands, is the most feared and challenging complication. Limited space through the incision, difficult handling and specific features of pulmonary vessels and circulation make the management of vascular injury a life-threatening situation. Prevention and preparation for the bleeding are the most effective tools for dealing with vascular injuries. With progressive exposure and training, there are several techniques for dealing from small to huge injuries that always begin with immediate control of the bleeding by direct compression, and undelayed conversion to open thoracotomy if not achieved (Figure 8).

Acknowledgments
The authors would like to thank the global thoracic surgeon community, which is working hard in the 21st century, struggling with the intrinsic difficulties of daily dealing with pulmonary vessel dissection to provide our patients with the best care available decreasing surgical invasiveness, anesthetic aggressiveness and preserving pulmonary parenchyma.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Video-Assisted Thoracic Surgery for the series “Advanced Uniportal VATS”. The article has undergone external peer review.
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-23-1/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-23-1/coif). The series “Advanced Uniportal VATS” was commissioned by the editorial office without any funding or sponsorship. CG served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lewis RJ, Caccavale RJ, Sisler GE, et al. Video-assisted thoracic surgical resection of malignant lung tumors. J Thorac Cardiovasc Surg 1992;104:1679-85; discussion 1685-7. [Crossref] [PubMed]
- Murakawa T, Ichinose J, Hino H, et al. Long-term outcomes of open and video-assisted thoracoscopic lung lobectomy for the treatment of early stage non-small cell lung cancer are similar: a propensity-matched study. World J Surg 2015;39:1084-91. [Crossref] [PubMed]
- Nwogu CE, D'Cunha J, Pang H, et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg 2015;99:399-405. [Crossref] [PubMed]
- Cao C, Zhu ZH, Yan TD, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small-cell lung cancer: a propensity score analysis based on a multi-institutional registry. Eur J Cardiothorac Surg 2013;44:849-54. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- ESTS Database Annual Report 2020. Silver Book 2020.
- Sawada S, Komori E, Yamashita M. Evaluation of video-assisted thoracoscopic surgery lobectomy requiring emergency conversion to thoracotomy. Eur J Cardiothorac Surg 2009;36:487-90. [Crossref] [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Wei S, Chen N, Liu C, et al. Does single-port video-assisted thoracic lobectomy have favorable perioperative results for non-small cell lung cancer compared with multi-port approach? A systematic review and meta-analysis. Video-assist Thorac Surg 2017;2:33. [Crossref]
- Abouarab AA, Rahouma M, Kamel M, et al. Single Versus Multi-Incisional Video-Assisted Thoracic Surgery: A Systematic Review and Meta-analysis. J Laparoendosc Adv Surg Tech A 2018;28:174-85. [Crossref] [PubMed]
- Numajiri K, Matsuura N, Igai H, et al. Uniportal thoracoscopic pulmonary segmentectomy provides good perioperative results and early postoperative recovery. J Thorac Dis 2022;14:2908-16. [Crossref] [PubMed]
- McKenna RJ Jr. Complications and learning curves for video-assisted thoracic surgery lobectomy. Thorac Surg Clin 2008;18:275-80. [Crossref] [PubMed]
- Hoksch B, Ablassmaier B, Walter M, et al. Complication rate after thoracoscopic and conventional lobectomy. Zentralbl Chir 2003;128:106-10. [Crossref] [PubMed]
- Berry MF. Pulmonary Artery Bleeding During Video-Assisted Thoracoscopic Surgery: Intraoperative Bleeding and Control. Thorac Surg Clin 2015;25:239-47. [Crossref] [PubMed]
- Igai H, Kamiyoshihara M, Ibe T, et al. Troubleshooting for bleeding in thoracoscopic anatomic pulmonary resection. Asian Cardiovasc Thorac Ann 2017;25:35-40. [Crossref] [PubMed]
- Vallance A, Tcherveniakov P, Bogdan C, et al. The evolution of intraoperative conversion in video assisted thoracoscopic lobectomy. Ann R Coll Surg Engl 2017;99:129-33. [Crossref] [PubMed]
- Miyazaki T, Yamasaki N, Tsuchiya T, et al. Management of unexpected intraoperative bleeding during thoracoscopic pulmonary resection: a single institutional experience. Surg Today 2016;46:901-7. [Crossref] [PubMed]
- Decaluwe H, Petersen RH, Hansen H, et al. Major intraoperative complications during video-assisted thoracoscopic anatomical lung resections: an intention-to-treat analysis. Eur J Cardiothorac Surg 2015;48:588-98; discussion 599. [Crossref] [PubMed]
- Yamashita S, Tokuishi K, Moroga T, et al. Totally thoracoscopic surgery and troubleshooting for bleeding in non-small cell lung cancer. Ann Thorac Surg 2013;95:994-9. [Crossref] [PubMed]
- Mei J, Pu Q, Liao H, et al. A novel method for troubleshooting vascular injury during anatomic thoracoscopic pulmonary resection without conversion to thoracotomy. Surg Endosc 2013;27:530-7. [Crossref] [PubMed]
- Vieira A, Bourdages-Pageau E, Kennedy K, et al. The learning curve on uniportal video-assisted thoracic surgery: An analysis of proficiency. J Thorac Cardiovasc Surg 2020;159:2487-2495.e2. [Crossref] [PubMed]
- Perna V, Carvajal AF, Torrecilla JA, et al. Uniportal video-assisted thoracoscopic lobectomy versus other video-assisted thoracoscopic lobectomy techniques: a randomized study. Eur J Cardiothorac Surg 2016;50:411-5. [Crossref] [PubMed]
- Martin-Ucar AE, Aragon J, Bolufer Nadal S, et al. The influence of prior multiport experience on the learning curve for single-port thoracoscopic lobectomy: a multicentre comparative study. Eur J Cardiothorac Surg 2017;51:1183-7. [Crossref] [PubMed]
- Bourdages-Pageau E, Vieira A, Lacasse Y, et al. Outcomes of Uniportal vs Multiportal Video-Assisted Thoracoscopic Lobectomy. Semin Thorac Cardiovasc Surg 2020;32:145-51. [Crossref] [PubMed]
- Yan Y, Huang Q, Han H, et al. Uniportal versus multiportal video-assisted thoracoscopic anatomical resection for NSCLC: a meta-analysis. J Cardiothorac Surg 2020;15:238. [Crossref] [PubMed]
- Magouliotis DE, Fergadi MP, Spiliopoulos K, et al. Uniportal Versus Multiportal Video-Assisted Thoracoscopic Lobectomy for Lung Cancer: An Updated Meta-analysis. Lung 2021;199:43-53. Erratum in: Lung 2021;199:235. [Crossref] [PubMed]
- Wu CF, de la Mercedes T, Fernandez R, et al. Management of intra-operative major bleeding during single-port video-assisted thoracoscopic anatomic resection: two-center experience. Surg Endosc 2019;33:1880-9. [Crossref] [PubMed]
- Liu L, Mei J, He J, et al. International expert consensus on the management of bleeding during VATS lung surgery. Ann Transl Med 2019;7:712. [Crossref] [PubMed]
- Takahashi Y, Soh J, Shien K, et al. Fibrosis or Necrosis in Resected Lymph Node Indicate Metastasis Before Chemoradiotherapy in Lung Cancer Patients. Anticancer Res 2020;40:4419-23. [Crossref] [PubMed]
- Provencio M, Serna-Blasco R, Nadal E, et al. Overall Survival and Biomarker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non-Small-Cell Lung Cancer (NADIM phase II trial). J Clin Oncol 2022;40:2924-33. [Crossref] [PubMed]
- Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 2022;386:1973-85. [Crossref] [PubMed]
- Cascone T, William WN Jr, Weissferdt A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med 2021;27:504-14. [Crossref] [PubMed]
- Gonzalez-Rivas D, Stupnik T, Fernandez R, et al. Intraoperative bleeding control by uniportal video-assisted thoracoscopic surgery. Eur J Cardiothorac Surg 2016;49:i17-24. [PubMed]
- Iwasaki A, Okabayashi K, Shirakusa T. A model to assist training in thoracoscopic surgery. Interact Cardiovasc Thorac Surg 2003;2:697-701. [Crossref] [PubMed]
- Haidari TA, Bjerrum F, Hansen HJ, et al. Simulation-based VATS resection of the five lung lobes: a technical skills test. Surg Endosc 2022;36:1234-42. [Crossref] [PubMed]
- Chen Z, Zhang Y, Yan Z, et al. Artificial intelligence assisted display in thoracic surgery: development and possibilities. J Thorac Dis 2021;13:6994-7005. [Crossref] [PubMed]
- CTSNet [Internet]. Available online: www.ctsnet.org
- WebSurg [Internet]. Available online: https://websurg.com/
- Youtube [Internet]. Available online: https://youtube.com/
- Manabe T, Kuwata T, Taira A, et al. Preoperative 4D-CT Provided Helpful Evaluation of Aorta Invasion of Left Lower Lobe Lung Cancer. J UOEH 2020;42:365-9. [Crossref] [PubMed]
- Ma Q, Bao T, Zhang H, et al. Anatomical video-assisted thoracoscopic surgery segmentectomies based on the three-dimensional reformation images. J Vis Surg 2017;3:21. [Crossref] [PubMed]
- Kato H, Oizumi H, Suzuki J, et al. Thoracoscopic anatomical lung segmentectomy using 3D computed tomography simulation without tumour markings for non-palpable and non-visualized small lung nodules. Interact Cardiovasc Thorac Surg 2017;25:434-41. [Crossref] [PubMed]
- Hagiwara M, Shimada Y, Kato Y, et al. High-quality 3-dimensional image simulation for pulmonary lobectomy and segmentectomy: results of preoperative assessment of pulmonary vessels and short-term surgical outcomes in consecutive patients undergoing video-assisted thoracic surgery. Eur J Cardiothorac Surg 2014;46:e120-6. [Crossref] [PubMed]
- Navarro-Martínez J, Gálvez C, Rivera-Cogollos MJ, et al. Intraoperative crisis resource management during a non-intubated video-assisted thoracoscopic surgery. Ann Transl Med 2015;3:111. [PubMed]
- Safdie FM, Sanchez MV, Sarkaria IS. Prevention and management of intraoperative crisis in VATS and open chest surgery: how to avoid emergency conversion. J Vis Surg 2017;3:87. [Crossref] [PubMed]
- Villa M, Sarkaria IS. Great Vessel Injury in Thoracic Surgery. Thorac Surg Clin 2015;25:261-78. [Crossref] [PubMed]
- Hsin MK, Yim AP. Management of complications of minimally invasive thoracic surgery. Respirology 2010;15:6-18. [Crossref] [PubMed]
- Guo C, Mei J, Ma L, et al. Handling vascular bleeding without conversion during video-assisted thoracoscopic surgery major pulmonary resection. Ann Transl Med 2018;6:363. [Crossref] [PubMed]
- Kohno T. Management of complications in thoracoscopic surgery. J Thorac Dis 2018;10:S1620-3. [Crossref] [PubMed]
- Xiao ZL, Mei JD, Pu Q, et al. Technical strategy for dealing with bleeding during thoracoscopic lung surgery. Ann Cardiothorac Surg 2014;3:213-5. [PubMed]
Cite this article as: Galvez C, Maroto S, Sebastian L, Lirio F, del Campo J, Sesma J, Bolufer S. Bleeding control during uniportal video-assisted thoracic surgery without conversion: evidence and technical aspects. Video-assist Thorac Surg 2023;8:33.

















