Uniportal, robot-assisted and uniportal robot-assisted subxiphoid thymectomy with CO2 insufflation
Highlight box
Surgical highlights
• In uniportal or uniportal robot assisted approach, the port is inserted into a single-port wound.
• In robotic multiport thymectomy, the ports are inserted on the right sixth intercostal mid-axillary line, right sixth intercostal anterior axillary line, and left sixth intercostal anterior axillary line.
• CO2 insufflation at 8 mmHg.
• Bilateral mediastinal pleural incisions to open the bilateral thoracic cavities.
What is known and what is new?
• Thymectomy via the subxiphoid approach is an excellent technique for both surgeons and patients because the operative field in the neck region is secured, bilateral phrenic nerve identification is possible, cosmetic outcomes are superior, and pain is minimal.
What is the implication, and what should change now?
• The standard surgical method for thymectomy should shift from the intercostal lateral approach to the subxiphoid approach with better visibility and operability.
Introduction
At present, thymectomy is performed endoscopically. The primary approaches for performing endoscopic thymectomy are the transcervical, lateral intercostal, and subxiphoid approaches (1-5). At the same time, robot-assisted thymectomy is also being performed (4,6). Regardless of the approach used, the space behind the sternum requires expansion to resect the thymus, which is located in the narrow space between the sternum and mediastinum containing the heart. Reported methods of expanding the space behind the sternum include lifting the thoracic region with a steel wire passed subcutaneously, sternum lifting, and carbon dioxide (CO2) insufflation. This study reports the procedures we currently use, including subxiphoid uniportal thymectomy (SUT) and subxiphoid robotic thymectomy (SRT) using CO2 insufflation, as well as subxiphoid uniportal robot-assisted thymectomy (SURT) using the da Vinci Xi surgical system.
Preoperative preparations and requirements
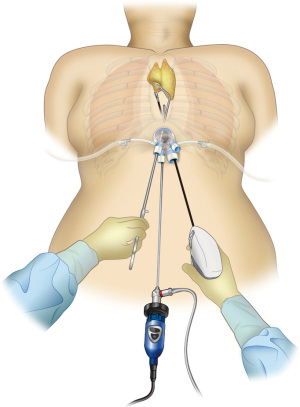
To perform SUT (Figure 1), tissue-grasping forceps angled at 50 degrees for thymus grasping and slightly curved tissue-grasping forceps for cervical manipulations (Geister, Tuutlingten, Germany) are needed (Figure 2). The port for this surgical procedure should have a single-port design that permits CO2 insufflation. To date, we have used many different ports, but we feel that Alnote Lapsingle (Sejong Medical Co., Paju, Korea) has the best operability for subxiphoid thymectomy with CO2 insufflation.

The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Fujita Health University (approval numbers HM20-438 and HM22-572) and informed consent was obtained from all individual participants.
Surgical procedure
Making skin incision until the bilateral thoracic cavities are exposed
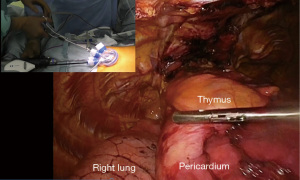
A skin incision of 3 cm in length located 1 cm caudal to the xiphoid process is made. Horizontal incision is made in SUT and vertical incision is made in SRT to make it easier for the assistant to insert suction tube and forceps through the inferior border of the wound. SURT is performed by making a horizontal incision, which improves the arm mobility. The linea alba of the rectus abdominis is separated from the xiphoid process in a lateral direction; therefore, is dissected approximately 1 cm caudally in a vertical direction. The practitioner inserts a finger behind the sternum and blindly separates the thymus from the posterior surface of the sternum. Subsequently, the finger is moved caudally to blindly separate the posterior surface of the linea alba avoiding breaking the peritoneum tear. Surgery can be continued even if the peritoneum breaks; however, operative manipulations can be slightly difficult when CO2 enters the abdominal cavity. Alnote Lapsingle (Sejong Medical Co., Paju, Korea), a port for single-port surgery, is inserted in a separate space and a CO2 insufflation device is connected. CO2 insufflation devices are sold by various companies; however, when suction is performed using these devices, there is a reduction in intrapleural pressure and causes the lungs to expand, which disturbs the visual field. However, while performing aspiration using the AirSeal® system (CONMED, Largo, FL, USA), which maintains constant air supply pressure, the visual field disturbance due to expansion of the lungs is resolved and surgery can be safely performed. A dedicated port is needed while using AirSeal; therefore, a port for AirSeal is inserted into the Alnote Lapsingle miniport. Next, a 30-degree angled rigid endoscope is inserted and CO2 insufflation is commenced at 8 mmHg. CO2 insufflation displaces dorsally the pericardium and laterally the bilateral mediastinal pleura, thereby enlarging the space behind the sternum. While detaching the thymus from the posterior surface of the sternum using an energy device, CO2 insufflation enlarges the space behind the sternum. A hole is made in the bilateral mediastinal pleura by opening and closing the tip of the energy device, which exposes the bilateral pleural spaces and separates the mediastinal pleura from the posterior surface of the sternum. CO2 insufflation displaces the lungs dorsally while maintaining ventilation; therefore, single-lung ventilation is unnecessary. One trick for smoothly performing this surgery is to avoid using positive end-expiratory pressure (PEEP) in the artificial ventilator settings. The lungs expand bilaterally when PEEP is used, which disturbs the visual field. If the blood CO2 concentration increases due to hypoventilation, it can be managed by increasing the ventilation frequency. Sufficient space for surgical manipulations behind the sternum can be achieved by CO2 insufflation (Figure 3).
SUT
Initially, the most caudal side of the thymus is grasped with the tissue-grasping forceps angled at 50 degrees and lifted to detach the thymus from the pericardium. Once the layer of the pericardium is visible, the inferior pole of the thymus is detached from the pericardium layer. The location of the bilateral phrenic nerves is confirmed, and the thymus is detached from the pericardium at 1 cm medially to the bilateral phrenic nerves to avoid injury. The thymus is detached from the left side of the left brachiocephalic vein by opening and closing the jaws while lifting the tip of the energy device to slice the surface of the thymus near the brachiocephalic vein. Dissecting only the detached portion is a way to avoid cutting the left brachiocephalic vein. Once the left brachiocephalic vein is exposed, the superior pole of the left thymus is detached from the cranially located trachea. On the right side of the left brachiocephalic vein, the thymus is detached from the pericardium at 1 cm medially to the right phrenic nerve. As the detachment proceeds cranially, the superior vena cava can be identified. Therefore, the left brachiocephalic vein will naturally be exposed upon detaching the thymus to the cranial side until the superior vena cava is located. As the detachment proceeds cranially and reaches near the thyroid gland, the right brachiocephalic vein, the brachiocephalic artery, and inferior thyroid vein are located. The left and right superior poles of the thymus are grasped with slightly curved tissue-grasping forceps, and a good view of the visual field of the cranial side of the right brachiocephalic vein is achieved upon pulling the forceps caudally. Adipose tissue including the bilateral superior poles of the thymus is detached from the thyroid on the cranial side, the right brachiocephalic vein on the right side, the superior border of the left brachiocephalic vein on the left side, and the trachea and brachiocephalic artery on the mediastinal side. Lastly, the thymic vein is dissected, thereby completing total thymectomy. Inside the thoracic cavity, the thymus is placed into a plastic bag and removed via the subxiphoid wound. After removing the air within the thoracic cavity using a drain, the drain is removed in the operating room (Video 1).
SRT
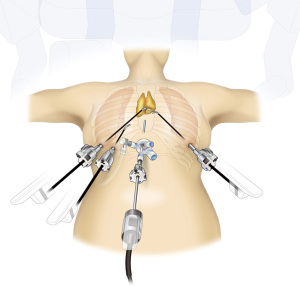
Once the mediastinal pleura are exposed bilaterally, an 8 mm port is inserted on the right sixth intercostal mid-axillary line, right sixth intercostal anterior axillary line, and left sixth intercostal anterior axillary line. In the supine position, the left side of the chest is near the heart; care should be taken to avoid possible heart injury while inserting and removing the port and tools. To avoid heart injury, only one port is inserted in the left side. A shortcoming of the visual field of the camera inserted via a subxiphoid is the difficulty in identifying adipose tissue in the left pericardium. Identification of the tissue can be achieved by grasping the thymus and firmly pulling it to the right side. For easy verification of left phrenic nerve position, the following process can be followed: (I) insert an intercostal port into the lateral chest; (II) before docking the robot, insert a camera via the port in the left sixth intercostal space; (III) tilt the bed to place the patient in the right semi-lateral position, thereby moving the heart to the right by gravity, which enlarges the space of the left thoracic cavity; and (IV) verify the position of the left phrenic nerve across its entire length. Marking is performed using gentian violet at approximately 1 cm anterior to the left phrenic nerve, after which the bed is returned to the supine position. Thereafter, the robot is docked and a camera is inserted via the subxiphoid wound; however, the cranial border of the wound is targeted to ensure space for the assistant to insert instruments in the caudal side in the inferior border of the wound. In the port located in the right sixth intercostal mid-axillary line, Cadiere forceps are inserted and used as a retraction arm (Figure 4). For accessing the port of the right anterior axillary line, fenestrated bipolar forceps are used and the surgery is performed after inserting SynchroSeal via the left lateral chest port. The left inferior pole of the thymus is dissected along the line of the gentian violet marking. For performing surgical manipulations, a camera is inserted via the right lateral chest from the inferior pole of the right thymus near the right brachiocephalic vein. Therefore, surgery is performed to observe the right phrenic nerve and diaphragm using fenestrated bipolar forceps inserted into the port of the right sixth intercostal mid-axillary line, camera inserted via the port of the right sixth intercostal anterior axillary line, SynchroSeal (held in the practitioner’s right hand) inserted via the subxiphoid wound, and Cadiere forceps inserted via the port of the left lateral chest as a retraction arm (Video 2). Although a good assistant can develop the operative field without using an arm retraction, using an arm retraction makes it possible to pull the thymus in the operator’s desired direction and create a good field of view in a narrow and limited space. The abundance of the thymus and surrounding adipose tissue complicated any approach in severely obese patients. In the subxiphoid approach, the most proximal adipose tissue is first dissected from the pericardium to identify the pericardial layers. Thereafter, the arm retraction is used to retract the thymus in the appropriate direction to secure the visual field, and the pericardial layer is dissected cranially.
SURT using da Vinci Xi system
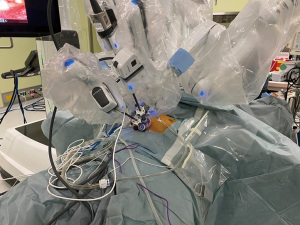
In recent years, uniportal robot-assisted thoracoscopic surgery (uniportal RATS) using the da Vinci Xi system has been performed (7,8). SURT performed using the da Vinci Xi system have also been reported. Similar to the aforementioned uniportal thymectomy, the robot is docked after opening the bilateral mediastinal pleura for exposing the bilateral thoracic cavities. The cranial portion of the port interferes in the port head, which can be prevented by changing the depth of port insertion. With a central focus on the camera, the forceps held by the practitioner are inserted in the left and right side of the camera. We operated the arms within the thoracic cavity in a parallel manner (Figure 5). Fenestrated forceps or tip-up grasper is primarily held in the left hand; whereas, SynchroSeal is held in the right hand. A case of thymoma with a cyst is presented in Video 3.
Discussion
Insufflation of CO2 into the mediastinum displaces the pericardium and lungs dorsally and enlarges the space behind the sternum. While performing SUT using CO2 insufflation, concurrent sternum was lifted using a subcutaneously passed steel wire; however, the procedure was not used after a few cases owing to the insufficient space created by CO2 insufflation alone. The advantages of CO2 insufflation are that the organs and tissues are not injured by instruments, surgical manipulations can be performed with bilateral ventilation, and a wide surgical space can be created upon displacing the pericardium and lungs. The disadvantage of this method is the lack of ventilation that can cause hypercapnia; however, this can be resolved by increasing the ventilation frequency.
When thymectomy is performed endoscopically, many institutions are thought to adopt a lateral chest intercostal approach; however, as the position of the contralateral phrenic nerve cannot be confirmed in this approach, the phrenic nerve may be damaged. Furthermore, the shortcoming of using this approach when performing extended thymectomy for myasthenia gravis is the inadequate resection of thymus near the contralateral phrenic nerve. Moreover, the lateral chest approach can entail risks when a tumor is near the left brachiocephalic vein, or when here is bleeding from the brachiocephalic vein that needs clamping to ensure the contralateral left brachiocephalic vein. These risks can be attributed to the lack of visibility of the contralateral brachiocephalic vein due to the tumor and manipulations that cause compression in the visual field. In contrast, the visual field from the body midline below the xiphoid process enables observation of the left and right phrenic nerves and the entire left brachiocephalic vein in the subxiphoid approach. Therefore, allowing surgery to be performed safely by taping the left brachiocephalic vein proximally and distally. This procedure is performed in the supine position and is easy to change to median sternotomy.
SUT is the most minimally invasive procedure for thymectomy because it does not pass through the intercostal space; therefore, there is no intercostal nerve damage and post-thoracotomy pain (9). There are reports of the procedure performed under local anesthesia. This procedure is the least invasive for patients, and it is used in the cases of myasthenia gravis and anterior mediastinal tumors including thymoma that do not touch the left brachiocephalic vein and left and right phrenic nerves. When bleeding occurs during SUT, for instance from the left brachiocephalic vein, an additional port is placed and a valved port is inserted to avoid drops in intrathoracic pressure generated due to influx of CO2. When TachoSil® (Takeda Austria GmbH, Linz, Austria) is inserted for achieving compression hemostasis, it should be ensured that the port has a valve; if TachoSil® is inserted as is, it will be torn. Insertion after wrapping with small gauze for endoscopic surgery enables TachoSil® to be inserted without tearing, even the valved ports.
SRT is used if there is a tumor located adjacent to the left brachiocephalic vein, cranially to the left brachiocephalic vein, or laterally to the phrenic nerves. If surgery is performed at an institution where SUT is not performed, then SRT has the best operability with a wide range of surgical indications, enabling suture insertion and end-to-end anastomosis of blood vessels. We have used this approach for concurrent resection of the superior vena cava and bypass surgery using a vascular graft for right brachiocephalic vein, right auricular appendage bypass and right brachiocephalic vein, and superior vena cava bypass. SRT is a desirable approach as it offers good visual field and robot operability.
SURT is an approach that combines the minimal invasiveness of the single-port surgery and good operability of robotic surgery to resolve the need for increased number of ports, which is one shortcoming of the robotic surgery. However, as the instruments are inserted into a single-port wound of less than 4 cm, which is much smaller than the normally required distance between arms (6 cm), interference between instruments can pose a problem. Further techniques are needed that reduce the range of motion of the left and right arms in SURT. Thymectomy using the da Vinci SP system, which is a robot system for single-port surgery, is being performed; however, we have no experience of SURT, as it is not yet available in Japan. One shortcoming of robotic surgery is its high-cost burden than endoscopic surgery performed manually, and there is no evidence to demonstrate its advantage for the patients, which is also true for SURT. It must be clearly demonstrated that the use of a robot is more advantageous than the manual endoscopic surgery. We believe that one advantage of robotic surgery is that it helps in performing difficult surgery that was so far considered impossible without thoracotomy. Future improvement in operability should demonstrate that its benefits outweigh the higher costs. We believe that there are ample possibilities that single-port robotic surgery could serve as the standard surgery for thymectomy.
Conclusions
SUT is the most minimally invasive procedure for thymectomy because it does not pass through the intercostal space. SRT is a desirable approach as it offers good visual field and robot operability. SRT has the best operability with a wide range of surgical indications. SURT is an approach that combines the minimal invasiveness of the single-port surgery and good operability of robotic surgery to resolve the need for increased number of ports, which is one shortcoming of the robotic surgery. We believe that there are ample possibilities that single-port robotic surgery could serve as the standard surgery for thymectomy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Carlos Galvez Munoz and Paula A. Ugalde Figueroa) for the series “Advanced Uniportal VATS” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-22-55/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-22-55/coif). The series “Advanced Uniportal VATS” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Fujita Health University (approval numbers HM20-438 and HM22-572) and informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Detterbeck FC, Kim AW, Zielinski M. Looking in from above and up from below: new vistas in thoracic surgery. Innovations (Phila) 2012;7:161-4. [Crossref] [PubMed]
- Cooper JD, Al-Jilaihawa AN, Pearson FG, et al. An improved technique to facilitate transcervical thymectomy for myasthenia gravis. Ann Thorac Surg 1988;45:242-7. [Crossref] [PubMed]
- Landreneau RJ, Dowling RD, Castillo WM, et al. Thoracoscopic resection of an anterior mediastinal tumor. Ann Thorac Surg 1992;54:142-4. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Operative techniques in robotic thoracic surgery for inferior or posterior mediastinal pathology. J Thorac Cardiovasc Surg 2012;143:1138-43. [Crossref] [PubMed]
- Suda T, Sugimura H, Tochii D, et al. Single-port thymectomy through an infrasternal approach. Ann Thorac Surg 2012;93:334-6. [Crossref] [PubMed]
- Suda T, Tochii D, Tochii S, et al. Trans-subxiphoid robotic thymectomy. Interact Cardiovasc Thorac Surg 2015;20:669-71. [Crossref] [PubMed]
- Yang Y, Song L, Huang J, et al. A uniportal right upper lobectomy by three-arm robotic-assisted thoracoscopic surgery using the da Vinci (Xi) Surgical System in the treatment of early-stage lung cancer. Transl Lung Cancer Res 2021;10:1571-5. [Crossref] [PubMed]
- Gonzalez-Rivas D, Bosinceanu M, Motas N, et al. Uniportal robotic-assisted thoracic surgery for lung resections. Eur J Cardiothorac Surg 2022;62:ezac410.
- Suda T, Hachimaru A, Tochii D, et al. Video-assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results. Eur J Cardiothorac Surg 2016;49:i54-i58. [PubMed]
Cite this article as: Suda T, Nagano H, Negi T, Tochii D, Tochii S. Uniportal, robot-assisted and uniportal robot-assisted subxiphoid thymectomy with CO2 insufflation. Video-assist Thorac Surg 2023;8:27.