Posterior approach for uniportal video-assisted thoracic surgery resection of lung segments: surgical technique
Highlight box
Surgical highlights
• Anterior incision in the IV-V intercostal space on the media-axillary line.
• Dissection within the fissure to locate the pulmonary artery and its branches for the specific lung segment.
• Identification of the vein for the apico-posterior segments by retracting the lung anteriorly.
• Alternatively, resection of the segment parenchyma without prior dissection of the segmental vein.
What is conventional and what is novel/modified?
• Posterior incision, specifically in the triangle of auscultation.
• Dissection of the broncho-vascular structure outside of the fissure.
• Avoidance of fissure dissection by undermining and resecting the fissure with a stapler.
• Enhanced accessibility to the veins.
What is the implication, and what should change now?
• The posterior approach offers easier management of posterior lung segments.
• Consideration of the modified technique for resections involving posterior segments.
• Further exploration and evaluation of the implications and benefits of the posterior approach in uniportal video-assisted thoracic surgery.
Introduction
Uniportal video-assisted thoracoscopic surgery (uVATS) has emerged as an innovative approach for the treatment of lung cancers (1). This approach is less invasive than traditional open thoracotomy and provides better visualization, higher magnification, and better ergonomics for the surgeon. The posterior approach is a modification of the standard uVATS and can be used for resection of the posterior lung segments. The purpose of this article is to present a detailed surgical technique for the posterior approach of uniportal VATS for lung resections. A description of this approach’s feasibility, benefits, and drawbecks can be found elsewhere (2).
Preoperative preparations and requirements
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Rhineland-Palatinate (No. 2021-15979) and individual consent for this retrospective analysis was waived.
There are no specific preoperative preparations or requirements needed for this surgical procedure, except for the usual protocols. However, the positioning of the patient on the surgical table is critical due to the narrow intercostal spaces. To optimize the positioning, the patient should be slightly bent forward to expose the triangle of auscultation. Additionally, the surgical table should be adjusted to bend precisely at the location of the incision for maximum benefit.
Step-by-step description
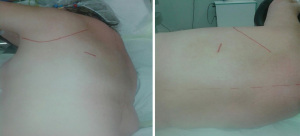
The incision is placed a few centimeters caudal and posterior to the apex of the scapula in the triangle of auscultation (3,4). The length of the incision is about 3–4 cm. Although both surgeons and assistants approach from behind, they may vary their positions during the operation based on anatomical factors like chest size, diaphragm position, and incision caudality relative to intrapleural anatomy, but normally do not cross over on the other side (Figure 1).
Cameras can be placed in any convenient position—the position of the instruments is not critical.
Left-sided resection
The hilum on the left differs from the right side, with the pulmonary artery (PA) easily detected, approached, and managed. A fissureless “fissure first” technique may prove useful in tunneling between the PA and a fissure (5).
Apico-posterior segment of the left lower lobe (S6)
The apico-posterior arterial branch (A6) is readily exposed, it is relatively easier to clip or ligate it due to its close proximity. Nevertheless, stapling is my preferred method of addressing it being simple and safe, although it might seem daunting sometimes, especially when 30 mm loads are temporary absent.
In subsequent steps, a bronchus will always be found on caudally (on the left) of the PA, followed by a lower lung vein. Identifying the bronchus visually and palpatorily allows you to find the apico-posterior branch (B6) easily. Usually, a lymph node located at this bifurcation (between B6 and the basal group branch) can be moved aside, giving the B6 enough room to be encircled and eventually taken. Stapling and manual suturing are both options. If the later is the case, I personally prefer PDS 4–0 continuous sutures, as they are non-resorbable monofilament sutures.
An apico-posterior vein is always present immediately caudally (on the left) of the B6. If it were divided separately, as opposed to the division along with the lung parenchyma, this would provide a better and deeper entrance for stapling (Video 1).
There are several ways to determine the boundary of the S6, but I actually stick to two:
- Clamping of the segmental bronchus: As the insufflation of the lung is delayed sufficiently, the apicoposterior segment’s boundaries become evident.
- “Indocyanine green” (ICG) is an inexpensive but handy camera module. It functions as follows:
- Following the ligation of an artery of a particular segment (in this case the A6), the video device is switched to near-infrared (ICG) mode;
- Next, a colleague in anesthesiology is requested to dissolve up to 25 mg of ICG powder (VERDYE, Diagnostic Green Ltd., DE) in 10 mL of aqua and inject it into any vein available;
- After the injection, it is flushed with a simple saline solution, say 10–20 mL, to create a green landscape within the chest except for S6 due to a perfusion deficit caused by exclusion of segmental arterial blood supply (Video 2).
Left upper lobe segmentectomy S1+2 start at the same point as S6. Taking care of A1+A2c, the first apical arterial branch of the PA entering the fissure, is the first step (Video 3). From the apex of the hilum, the division of the A1+2a+b should follow. In practice, it’s more advantageous to go for the V1+V2a-c, since it’s easy to distinguish, encircle, and divide with a stapler, thus exposing the A3 and A1+2a+b. The reason for this is that sometimes those branches have only one joint trunk. In case the small tributary vein V1+2d is present, it will be resected along with the parenchyma.
It is easy to approach the bronchus from the posterior, perhaps even easier than from the anterior. By means of bronchoscopy, it can be difficult to determine which bronchus is correct when a thicker clamp is placed on B1+2. The insufflation test could be very helpful though.
Right-sided resections
Using this approach, it is easy to access two segments on the right side: the posterior-apical segment of the lower lobe and the posterior segment of the upper lobe. In contrast to the left side, the PA here is not readily available, it must be exposed first.
An upper lobar bifurcation and a lymph node at position 11 between the two bronchi will be the first thing one encounters; despite not having to be particularly large, this lymph node can still be an obstacle to achieving a clear anatomical presentation.
Gentle handling should come without saying since it is followed immediately by two critical structures: the posterior segment vein (V2) and the PA. At this stage of surgery, they may be injured, potentially preventing a minimally invasive approach (and altering the dominant color of the operating field).
Apico-posterior segment of the lower lobe (S6) on the right appears similar to the one opposite it. Dissection of the lymph node nr.11 (located between upper lobe bronchus and bronchus intermedius) exposes the cranial border of its (segment) bronchus, which allows for easy detection and encirclement.
Considering this, and the fact that B6 is so easily resected without entering the fissure (Video 4), in general, it is the first structure to be addressed following anatomical S6 resection on this side.
By removing the B6, the apico-posterior segment artery (A6) becomes clearly apparent and can easily be accessed. In this case, the tunneling between the artery and the fissure could be followed by stapling the fissure, which allows A6’s entire circumference to be exposed, sometimes revealing its existence as two A6, before one of them emerges as a surprise (even just for the sake of the beautiful non-red scenery).
Once the A6 is divided, it is possible to either continue with further fissure division (tunneling-stapling) or with a division of the apico-posterior vein (V6), which is readily available and rather well exposed once the B6 is removed, so it may be taken even before the A6 is divided. If A6 is tackled first, however, the segment may be more mobile, making stapler placement easier.
Finally, the segment needs to be separated from the lung—the procedure for identifying borders on the opposite side still applies, with my personal preference still being an insufflation test using clamped bronchi and ICG.
Right upper lobe posterior segment (S2) goes along with a few specifics to be noted. First, opening up the fissure is not necessary to remove the segment bronchus (B2). Once the right upper bronchus is identified, a stump dissection along it will reveal a small bifurcation (B1&B2). B2 can then be encircled and eventually divided (Video 5).
Therefore, the posterior segmental artery (A2) may be visible, as well as the venous artery (V2). The fissure can either be opened up or tunneled after dissection of the position 11 lymph node and visualization of the interlobar PA. At this point, the exact location of the V2 can be determined. There are times (more often than not) when a small venous branch V3a emerges from the posterior part of the S3 and joins the venous trunk, usually behind the A2.
It should be preserved, despite the presence of a second V3b vessel leaving the anterior segment.
In addition, the segment must be resected—ICG tests are usually more accurate and convenient than insufflation tests since insufflation tests produces fast insufflated upper lobes with little chance of marking their borders. In the absence of an ICG and insufflation tests fail, it is necessary to apply anatomy knowledge.
However, there is another landmark at the virtual border between segments 2 and 3—the small V3a branch. The resection may begin here, with an angle of 60–90 degrees toward the center of the lobe. Under- or over-resection resulting from an imprecise direction may result in a smaller S1 and/or S3 areas, or even incomplete S2 removal (Video 6).
Lymphadenectomy
As station 7 lies deeper on the left side and in a tighter surrounding, this approach has a clear advantage over the classic variant, at least for those not familiar with the anterior hilar approach to this lymph node station. Having it directly in front of the entrance, surrounded by no obstacles (i.e., lung), makes exposing, dissecting, and extracting it easier.
Station 6 and 5 remain accessible, especially station 6. Posterior VATS has a disadvantage with regard to station 5—they are easily picked up, but not with ease, especially not in the deep (toward position 4), at least not without a lengthy dissection and a fair chance of causing hoarseness.
When it comes to removing station 7, the right side is virtually identical to the left, with the exception that it may even be easier. It is sometimes feasible to begin with it and give the lung more time to collapse, since lightly to moderate inflated lungs do not hinder the lymphadenectomy at this position.
The lymph nodes 2 and 4 are also easily accessible—it might be best to begin below the v.azygos (position 10), remove a typical lymph node at the junction of the vv.azygos and cava (which sometimes does not align with the classic approach), and push it bluntly cranial. Now that the pleura above the v.azygos has been opened, it is easier to remove fat tissue and numerous lymph nodes.
Below are a few peer-reviewed videos that can provide further insight into the surgery (6-9).
Postoperative considerations and tasks
There used to be concerns about increased postoperative pain due to narrow intercostal spaces, size and positioning of the chest tube, which is located at the back and cause discomfort for the patient. Additionally, there was a risk of the chest tube getting kinked, leading to further complications. However, such complications have not been observed, and the pain experienced by patients did not appear to be more prominent than usual, although it is, as always, rather difficult to interpret.
It is still advisable to remove the chest tube as soon as possible, usually within the first or second postoperative day, and consider using a smaller size (such as 18Ch). Early mobilization and enhanced recovery protocols may also be beneficial for the patient’s recovery.
Tips and pearls
For the posterior uniportal approach for anatomical lung resection:
- Position the patient properly.
- Place the incision in the triangle of auscultation, one to two fingers below the tip of the scapula, with orientation over the latissimus dorsi muscle fold.
- Begin with lymphadenectomy to allow the lung more time to deflate.
- On the left side, first locate the PA and follow it as it enters the fissure. Undermining the fissure can facilitate its division and expose the A6 branch. If pursuing A1/2 segmentectomy, the A2c branch should be the first to be divided.
- On the right side, first locate the B6, which is the very first branch following intermediate bronchus caudal and starts at the upper lobar carina.
- If pursuing S2, the B2 should be the first structure to be taken care of, without entering into the fissure. To place the stapler, retract the lung posteriorly/dorsally so that the stapler follows an imaginary line parallel to the vertebral column.
- Usage of an “angle adapter” (Figure 2), which allows for greater control over instruments in close proximity to the optic. The light cable runs parallel to the optic, rather than emerging at a 90-degree angle.
Discussion and conclusions
The posterior uniportal approach for anatomical lung resection appears to be both feasible and safe, although additional reports may be necessary to confirm this. Despite a few drawbacks, such as the narrowness of the intercostal space and the rather inconvenient exit of the chest tube, there are also several advantages, including surgical ease, rapid orientation, and control over critical anatomic structures. Additionally, an excellent overview and non-obstructive path toward subcarinal lymph nodes may provide further oncological benefits. It is possible that this approach will find a place in the armamentarium of thoracic surgeons, as it has with us.
With the advancement of medical technology, this approach is becoming even more feasible. The first 20 resections were performed using a 10 mm optic, but now a 5 mm optic is routinely utilized.
In conclusion, despite its few drawbacks, the posterior uniportal approach has proven to be useful in appropriate indications, even 7 years after its initial application. Its surgical usage is likely the main reason for its continued use.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Carlos Galvez Munoz and Paula A. Ugalde Figueroa) for the series “Advanced Uniportal VATS” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-22-50/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-22-50/coif). The series “Advanced Uniportal VATS” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Rhineland-Palatinate (No. 2021-15979) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Stamenovic D, Bostanci K, Messerschmidt A. Posterior uniportal video-assisted thoracoscopic surgery for anatomical lung resections. J Thorac Dis 2017;9:5261-6. [Crossref] [PubMed]
- Naidu BV, Rajesh PB. Relevant surgical anatomy of the chest wall. Thorac Surg Clin 2010;20:453-63. [Crossref] [PubMed]
- Sayeed RA, Darling GE. Surface anatomy and surface landmarks for thoracic surgery. Thorac Surg Clin 2007;17:449-61. v. [Crossref] [PubMed]
- Decaluwe H, Sokolow Y, Deryck F, et al. Thoracoscopic tunnel technique for anatomical lung resections: a 'fissure first, hilum last' approach with staplers in the fissureless patient. Interact Cardiovasc Thorac Surg 2015;21:2-7. [Crossref] [PubMed]
- Stamenovic D. Uniportal Posterior Approach for Videothoracoscopic Anatomical Resection of Apical Segment of the Left Lower Lobe. CTSNet 2016; Published 16 May 2016, accessed 02.02.2023.
- Stamenovic D. Uniportal Posterior Approach for Videothoracoscopic Anatomical Resection of Posterior Segment of the Right Upper Lobe. CTSNet 2017; Published 04 January 2017, accessed 02.02.2023.
- Stamenovic D, Messerschmidt A. Posterior uniportal video-assisted thoracoscopic surgery for resection of the apical segment of the right lower lobe followed by completion lobectomy. Interact Cardiovasc Thorac Surg 2017;24:644-5. [Crossref] [PubMed]
- Stamenovic D. Posterior Uniportal VATS for the Posterior Segment (S2) Nonanatomical Resection Followed by Lobectomy Major Bleeding Event. 2019; [Crossref]
Cite this article as: Stamenovic D. Posterior approach for uniportal video-assisted thoracic surgery resection of lung segments: surgical technique. Video-assist Thorac Surg 2023;8:26.