
Assessment of the educational approaches for robotic minimally invasive esophagectomy
Introduction
Since the first large series on minimally invasive esophagectomy (MIE) by Luketich et al. in 2003 (1), multiple techniques utilizing the minimally invasive or hybrid approach have been introduced. The improved morbidity, mortality and short hospital stay (2) with equivalent oncological outcomes (3,4), has led to the wide adaption of these techniques. Currently the number of MIE and robotic-assisted minimally invasive esophagectomy (RAMIE) have surpassed the number of open esophagectomy (OE) performed in the United States (2).
RAMIE offers a proficient approach for esophageal, lymph node and hiatal dissection, fluorescence technology to ascertain conduit perfusion and less challenging techniques for the intrathoracic anastomosis. In 2016 we reported our early experience with the Robotic intrathoracic anastomosis using Circular End-to-End stapler with 0% leak rate and no conversion to open procedures (5). Similar experience was reported by Okusanya in 23 cases performed by a single surgeon using a similar technique with 1 leak in a series of 23 cases (6). Other surgeons and institutions had reported the safety and feasibility of Robotic esophagectomy.
The first prospective trial comparing robotic-assisted minimally invasive thoraco-laparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer (The ROBOT trial) comparing RAMIE (n=54) and OE (n=55) via a modified McKeown approach (7) with a 26% relative reduction risk in the RAMIE group in overall postoperative complications and similar oncological outcomes. Interestingly both arms utilized a cervical anastomosis and had a leak rate of 20%.
The TIME (8), MIRO (9) and the ROBOT (7) trials have shown that esophagectomy done Thoracoscopically, Laparoscopically or Robotically have the advantage in quality of life (QOL) questionnaires, facilitating the return to normal life and increasing the probability to complete systemic adjuvant therapy. The discussion of the best approach for esophageal cancer resection is ongoing and beyond the scope of this article. We think that the best approach is the approach that is feasible and safe for depending on the surgeon’s experience and the patient characteristics.
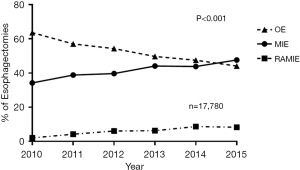
The wide adaptation of MIE and RAMIE (Figure 1) (2,10) necessitate the need for a thoughtful educational approach to prepare the next generation of surgeons to master the complexities of esophagectomy. This review will consider the factors that may help trainees and surgeons achieve proficiency and navigate their learning curve for RAMIE.
Understanding the learning curve
The Learning curve is defined as the number of operations that must be performed by a surgeon to achieve a steady level of performance (11). Implementation of robotic surgery in various surgical fields have encountered a learning curve including colectomy (12), gastrectomy (13) and hepatectomy (14). This has been documented even in experienced hands for MIE (15). In a series of 170 MIE, White et al. described an accumulative learning curve for one surgeon over 7 years. In the study period, the length of stay and 90 days readmission decreased by the end of the study. No leak and one conversion to OE were reported in the fourth quartile, reflecting the ability of surgeon to affect outcomes from the experiences they gain during the learning curve (15).
The learning curve for MIE and RAMIE has been described by intraoperative blood loss, number of harvested lymph nodes, operative time and learning associated morbidity (15-19). Most of the studies analyzing the learning curve reported single surgeon and institutional experience with significant heterogeneity in methodology, how groups were assigned, surgical approach and surgeons experience. There is no universal admissible number of cases to overcome the learning curve as surgeons differ in their training and abilities but it is inevitable that surgeons will encounter a learning curve morbidity while implementing new technology. We aim to review the available literature that may help surgeons and trainee flatten their steep curve.
It is important to note that the learning curve for RAMIE is multiphasic. Van der Sluis has reported this finding for a proctor and a newly introduced surgeon in 312 RAMIE using the cumulative sum (CUSUM) analysis (17). Three phases of learning curve were described for both surgeons. Phase 1 describe the cases the surgeon needs to reach a relative plateau of proficiency. Phase 2 represent increasing competency and phase 3 represent the surgeons approaching more difficult cases including more proximal tumors or advanced staging (cT4b). This has been also described by Kim et al. in a multicenter prospective trial for robotic gastrectomy (13).
Attaining proficiency
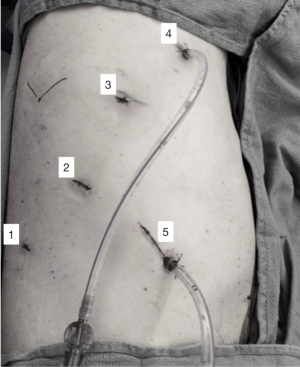
Attaining proficiency level for trainees or new robotic surgeons require few key elements. This includes patient selection and preparation for robotic surgery, trocar placement, robot docking, trouble shooting, operating room (OR) team efficiency, and emergency undocking scenarios (20). Error in docking, trocars positions and robotic positions can make RAMIE more challenging and can lead the surgeon to convert to an open or hybrid approach. Attending bedside robotic training can help avoid these pitfalls. Port placement for RAMIE has been described in multiple reports (21,22). Choice of port placement in the abdomen will facilitate good visualization of the conduit and the ability to perform a Kocher maneuver without the need to redock. In the chest, it is important to plan port placement to allow esophagus dissection, lymph node dissection as well allowing for an intrathoracic anastomosis while avoiding arm collision. Figure 2 shows our preferred thoracic port placement. This can be applicable to all robotic cases but it’s of great importance in RAMIE, as dissection and exposure can become challenging with improper docking or port placement. This is more relevant to RAMIE as inefficient port placement and docking can increase the operative time in an already complex and lengthy procedure. Port placement can be particularly challenging in patients who underwent previous jejunostomy placement due to the adhesions and poorly planned jejunostomy. We recommend planning to place all ports more cephalad to the jejunostomy if possible or redo the jejunostomy to improve exposure, hiatal dissection, and conduit creation.
Sarkaria et al. described the elemental keys to achieve proficiency in their first 100 RAMIE case (23). These elements include pre-program cadaveric study, alternating bedside, and console roles between 2 surgeons after first 50 cases, senior expertise, and graded teaching of the procedure for their residents and fellows. We believe that graded teaching helps trainees achieve proficiency at a single task before moving on to a more complex and challenging part of the operation.
A pathway to competency in robotic thoracic surgery was suggested by Cerfolio (24). This approach allows for skills progression while maintaining the team confidence and avoiding lengthy operation that hurt a surgeon’s reputation, utilizing a gradual pathway to progress toward more complex procedures, for example, in completing an esophagectomy in the chest without anastomosis before performing a complete RAMIE. Learning robotic procedures may be better achieved in a cumulative approach. This cumulative nature is an important part of our educational approach in robotics for our trainee. Robotic surgery is an important part of General Surgery training and some trainees are more versed with the principles of robotics than others. It is important to understand your trainee’s robotic experience. These discussions and feedback have better results and retention when done in advance or after the case.
The value of simulation
The competency training and simulator plays a critical role in learning robotics. It allows surgeons to master basic robotic skills that are applied in different portions of a RAMIE. The da Vinci Skills Simulator (Intuitive Surgical, Sinnyvale, CA, USA) using a da Vinci Console and MIMIC simulation technology offer some basic robotic skills simulation that have been validated and has the potential to increase psychomotor skills and platform familiarity (25,26). It is recommended to complete 19 of 30 simulation exercise with a score of higher than 80% for novice users (27). This training is often a part of a general surgery residency and sometimes even during a surgical rotation for medical students. With the scarcity and unavailability of robotic time, simulation can help trainees build muscle memory for suturing, tying and enforcing safe robotic principles. Transforming these skills into a complex procedure like RAMIE can be challenging, starting with novice steps (dividing gastrocolic omentum, hiatal dissection in the abdomen and opening the posterior pleura and dividing the azygous in the chest) can teach trainees visual feedback and tissue handling before progressing to more challenging parts.
Transitioning from simulation to handling real tissue can be more challenging in robotics. In an open esophagectomy, the attending surgeon can easily redirect and provide exposure to the trainee. In robotics, the surgeon sitting on the console has full control, and it will be hard to overcome any mistakes once they are made. One way to overcome this is the attending maintaining control of the third robotic arm to improve retraction and optimize exposure while the trainee improve their skills using instruments like the bipolar or stapling.
This transition is granular and can be guided by meeting certain milestones while progressing from novice steps to more technically demanding. It’s important for the mentor to provide transparent and constructive feedback in a non-stress environment after the operation. Our stepwise approach is listed in Figure 3. Once the trainee masters a task they move to the next level. Progression in these tasks is from low to high risk and with ascending technical difficulty not in the sequential order of the operation. The attending surgeon can optimize the exposure during the task or let the trainee obtain their exposure or work in less ideal situation to challenge their skills (28). The time given to complete each task depends on the complexity of the task and the progression made by the trainee.

Mitzman et al. has described their formal curriculum for robotic thoracic surgery (28). In their review they reported their approach to teaching trainee robotic lung resection utilizing simulation, a progressive approach for intra-op task and video-based review and coaching. In their review, the trainee masters novice steps like inferior pulmonary ligament take-down before eventually progressing to expert level task like fissure dissection.
No expert robotic courses or simulation module for RAMIE exist, but a model for robotic pulmonary resection exist and have been validated in 30 participants including novice users, intermediate level surgeons with experience in VATS lobectomy, as well as experts who have performed Robotic lobectomy (29). To our knowledge, no similar module is available for esophagectomy, and arranging a wet lab training for esophagectomy might be a solution for this inadequacy.
In our institution, we arrange an annual Robotic week to prepare the incoming new trainees on the basic robotic skills, emergency undocking scenarios, and an animal module for lung and esophagus surgery. This approach helps trainees familiarize themselves with these procedure in a low stress environment and also gives our faculty a chance to understand the trainee level of skills and advise them how to move forward.
The intrathoracic anastomosis remains the Achilles tendon of RAMIE, leading to the learning curve morbidity (16) which can be avoided in some scenarios. Fabian has described a simulation of Thoracoscopic Intrathoracic Anastomosis for MIE that can be replicated in similar fashion for RAMIE. Their model was validated in five trainees (30). A cervical esophagogastric anastomosis simulator was designed by Orringer et al. using a portable and low-cost training box (31) that can be valuable to teach this approach to trainee.
The problem and the solution, structured robotic training
In voluntary survey completed by recent graduates of thoracic surgery training, 61.5% reported discomfort with robotic esophageal operation (32). This demonstrates the need for a thoughtful approach to improve the current robotic training. This includes creating a validated systemic RAMIE curriculum to train the next generation of robotic esophageal surgeons.
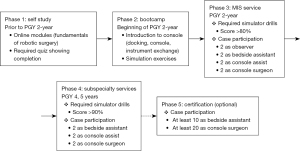
Raad et al. have proposed a structured curriculum divided into two stages: pre-clinical (PGY 2 and 3) and clinical (PGY 4–6) (33). In the pre-clinical years, residents complete online modules via the Fundamentals of Robotic Surgery or Intuitive Surgical Inc. da Vinci Surgery Online Community (Sunnyvale, CA, USA) and complete simulation modules achieving >85% score. The clinical years residents serve as the beside assistants and console surgeons in a graduated fashion. Once the residents show proficiency completing a task, they can progress to more complex components. This approach allows for training to occur during an extended period of time which decreases fatigue and enhances skill retention (34,35). Similar curriculum was suggested by Alicuben et al. for general surgery trainee (36) and is shown in Figure 4.
In Van Der Sluis et al. review of the learning curve in 312 RAMIE (17), they described the learning curve for two surgeons, a Proctor and Surgeon 2. Surgeon 2 was introduced to RAMIE after 20 procedures as bedside assistant and 5 observational cases. Fifteen RAMIE were performed under direct supervision by the proctor, who had performed 150 RAMIE at that time and achieved a steady performance. With this approach, surgeon 2 reached a steady performance within 13 months and after 24 cases which was a reduction of 66% in the number of cases and 76% in time compared to proctor learning curve. This highlights the importance of a structured and graduated curriculum to flatten the learning curve for RAMIE.
Recently, fourteen worldwide RAMIE experts were enrolled in a Delphi consensus project for elements of RAMIE training. This included 49 item questionnaire. Forty items reached consensus (20). All experts agreed to a standardized robotic curriculum that is divided into stages and includes baseline evaluation for assessment of training needs and bench marking to assess progress. Almost 93% agreed on the need for completion of e-learning and baseline evaluation before attending a robotic training course. 66% felt that the trainee’s ability to practice independently depends on achieving benchmarks and not a minimum number of cases. The authors also proposed the idea of RAMIE training centers that can be accredited via recognized education entity. This might be an important tool to help surgeons from lower caseload centers to adapt RAMIE, as the current curriculum are industry-based and not regulated by a scientific society. Although the proposed curriculums have not been validated, they offer the foundation for a dire need in a program that will facilitate the growth and adaption of RAMIE.
Conclusions
Robotic esophageal surgery continues to advance and gain more acceptance. This creates a need for a structured educational approach that helps trainee and future adaptors navigate the learning curve of these procedures. Establishing a RAMIE curriculum that includes a staged training in basics of robotics, bedside assistant and proctored cases will help surgeons attain proficiency and reduce the learning curve.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-22-46/coif). JOW serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from June 2022 to May 2024. JOW reports that he is a consultant for Intuitive, Ethicon, Medtronic, and Boston Scientific. Institution has research grant with Intuitive for robot. He also received payment for expert testimony for case reviews. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg 2003;238:486-94; discussion 494-5. [Crossref] [PubMed]
- Espinoza-Mercado F, Imai TA, Borgella JD, et al. Does the Approach Matter? Comparing Survival in Robotic, Minimally Invasive, and Open Esophagectomies. Ann Thorac Surg 2019;107:378-85. [Crossref] [PubMed]
- Dantoc M, Cox MR, Eslick GD. Evidence to support the use of minimally invasive esophagectomy for esophageal cancer: a meta-analysis. Arch Surg 2012;147:768-76. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Wee JO, Bravo-Iñiguez CE, Jaklitsch MT. Early Experience of Robot-Assisted Esophagectomy With Circular End-to-End Stapled Anastomosis. Ann Thorac Surg 2016;102:253-9. [Crossref] [PubMed]
- Okusanya OT, Sarkaria IS, Hess NR, et al. Robotic assisted minimally invasive esophagectomy (RAMIE): the University of Pittsburgh Medical Center initial experience. Ann Cardiothorac Surg 2017;6:179-85. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, van der Horst S, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer, a randomized controlled trial (ROBOT trial). Trials 2012;13:230. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- Mariette C, Markar S, Dabakuyo-Yonli TS, et al. Health-related Quality of Life Following Hybrid Minimally Invasive Versus Open Esophagectomy for Patients With Esophageal Cancer, Analysis of a Multicenter, Open-label, Randomized Phase III Controlled Trial: The MIRO Trial. Ann Surg 2020;271:1023-9. [Crossref] [PubMed]
- Kuppusamy MK, Low DEInternational Esodata Study Group (IESG). Evaluation of International Contemporary Operative Outcomes and Management Trends Associated With Esophagectomy: A 4-Year Study of >6000 Patients Using ECCG Definitions and the Online Esodata Database. Ann Surg 2022;275:515-25. [Crossref] [PubMed]
- Teplitz CJ. The learning curve deskbook: A reference guide to theory, calculations, and applications. Praeger, 1991.
- Bokhari MB, Patel CB, Ramos-Valadez DI, et al. Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc 2011;25:855-60. [Crossref] [PubMed]
- Kim MS, Kim WJ, Hyung WJ, et al. Comprehensive Learning Curve of Robotic Surgery: Discovery From a Multicenter Prospective Trial of Robotic Gastrectomy. Ann Surg 2021;273:949-56. [Crossref] [PubMed]
- Chen PD, Wu CY, Hu RH, et al. Robotic major hepatectomy: Is there a learning curve? Surgery 2017;161:642-9. [Crossref] [PubMed]
- White A, Kucukak S, Lee DN, et al. Ivor Lewis minimally invasive esophagectomy for esophageal cancer: An excellent operation that improves with experience. J Thorac Cardiovasc Surg 2019;157:783-9. [Crossref] [PubMed]
- Park S, Hyun K, Lee HJ, et al. A study of the learning curve for robotic oesophagectomy for oesophageal cancer. Eur J Cardiothorac Surg 2018;53:862-70. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, van der Horst S, et al. Learning Curve for Robot-Assisted Minimally Invasive Thoracoscopic Esophagectomy: Results From 312 Cases. Ann Thorac Surg 2018;106:264-71. [Crossref] [PubMed]
- Zhang X, Su Y, Yang Y, et al. Robot assisted esophagectomy for esophageal squamous cell carcinoma. J Thorac Dis 2018;10:3767-75. [Crossref] [PubMed]
- Zhang H, Chen L, Wang Z, et al. The Learning Curve for Robotic McKeown Esophagectomy in Patients With Esophageal Cancer. Ann Thorac Surg 2018;105:1024-30. [Crossref] [PubMed]
- Fuchs HF, Collins JW, Babic B, et al. Robotic-assisted minimally invasive esophagectomy (RAMIE) for esophageal cancer training curriculum-a worldwide Delphi consensus study. Dis Esophagus 2022;35:doab055. [Crossref] [PubMed]
- Shemmeri E, Wee JO. Robotics and minimally invasive esophageal surgery. Ann Transl Med 2021;9:898. [Crossref] [PubMed]
- Kuckelman J, Marshall MB, How I. Teach It: Robotic Assisted Minimally Invasive Esophagectomy (RAMIE). Foregut 2022;2:205-7. [Crossref]
- Sarkaria IS, Rizk NP, Grosser R, et al. Attaining Proficiency in Robotic-Assisted Minimally Invasive Esophagectomy While Maximizing Safety During Procedure Development. Innovations (Phila) 2016;11:268-73. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. How to teach robotic pulmonary resection. Semin Thorac Cardiovasc Surg 2013;25:76-82. [Crossref] [PubMed]
- Finnegan KT, Meraney AM, Staff I, et al. da Vinci Skills Simulator construct validation study: correlation of prior robotic experience with overall score and time score simulator performance. Urology 2012;80:330-5. [Crossref] [PubMed]
- Lerner MA, Ayalew M, Peine WJ, et al. Does training on a virtual reality robotic simulator improve performance on the da Vinci surgical system? J Endourol 2010;24:467-72. [Crossref] [PubMed]
- Rodriguez M, Ferrari-Light D, Wee JO, et al. The need for structured thoracic robotic training: the perspective of an American Association for Thoracic Surgery surgical robotic fellow. Ann Transl Med 2020;8:557. [Crossref] [PubMed]
- Mitzman B, Smith BK, Varghese TK Jr. Resident Training in Robotic Thoracic Surgery. Thorac Surg Clin 2023;33:25-32. [Crossref] [PubMed]
- Whittaker G, Aydin A, Raveendran S, et al. Validity assessment of a simulation module for robot-assisted thoracic lobectomy. Asian Cardiovasc Thorac Ann 2019;27:23-9. [Crossref] [PubMed]
- Fabian T, Glotzer OS, Bakhos CT. Construct validation: simulation of thoracoscopic intrathoracic anastomosis. JSLS 2015;19:e2015.00001.
- Orringer MB, Hennigar D, Lin J, et al. A novel cervical esophagogastric anastomosis simulator. J Thorac Cardiovasc Surg 2020;160:1598-607. [Crossref] [PubMed]
- Chu D, Vaporciyan AA, Iannettoni MD, et al. Are There Gaps in Current Thoracic Surgery Residency Training Programs? Ann Thorac Surg 2016;101:2350-5. [Crossref] [PubMed]
- Raad WN, Ayub A, Huang CY, et al. Robotic Thoracic Surgery Training for Residency Programs: A Position Paper for an Educational Curriculum. Innovations (Phila) 2018;13:417-22. [Crossref] [PubMed]
- Moulton CA, Dubrowski A, Macrae H, et al. Teaching surgical skills: what kind of practice makes perfect?: a randomized, controlled trial. Ann Surg 2006;244:400-9. [Crossref] [PubMed]
- Cannon-Bowers JA, Bowers C, Procci K. Optimizing learning in surgical simulations: guidelines from the science of learning and human performance. Surg Clin North Am 2010;90:583-603. [Crossref] [PubMed]
- Alicuben ET, Wightman SC, Shemanski KA, et al. Training residents in robotic thoracic surgery. J Thorac Dis 2021;13:6169-78. [Crossref] [PubMed]
Cite this article as: Abdallat M, Wee JO. Assessment of the educational approaches for robotic minimally invasive esophagectomy. Video-assist Thorac Surg 2023;8:14.


