
Experiences of broncho-pleural fistula after pulmonary resection in minimally invasive approach and open thoracotomy
Highlight box
Key findings
• It was considered there was no difference in the frequency of BPF between MIA and open thoracotomy. However, the incidence rate of BPF was low in MIA, it is possible that BPF may occur regardless of approach and procedure.
What is known and what is new?
• The most at risk of BPF was pneumonectomy.
• The BPF incidence by resection was 0.18% for lobectomy, 0.8% for segmentectomy, and 41.7% for pneumonectomy, whereas 0.29% for M-VATS, 0% for U-VATS, and 2.3% for RATS by surgical approach, which was similar to a previous report.
• There was no significant in the frequency of BPF between MIA and open thoracotomy except the cases of pneumonectomy.
What is the implication, and what should change now?
• Particular attention should be paid in multiple stapling procedures regarding the bronchus.
Introduction
Multi-port video-assisted thoracoscopic surgery (M-VATS) is the pioneer of minimally invasive approach (MIA) surgery for pulmonary resection with a lower complication rate than open thoracic surgery (1,2). The pulmonary resection approach has become more minimally invasive over the years, and nowadays, robot-assisted thoracoscopic surgery (RATS) and uniportal video-assisted surgery (U-VATS) are widely performed in addition to M-VATS. The safety and efficacy of RATS and U-VATS were similar to those of M-VATS (3,4), with an increasing number of surgeries.
MIA has a lower risk of postoperative complications than open thoracotomy (5-7), but a characteristic pulmonary resection complication of air leakage is experienced with MIA. The air leakage incidence is high for pleural-pulmonary fistulas, but they are often conservatively resolved and are rarely fatal. Conversely, broncho-pleural fistula (BPF) is unlikely to spontaneously heal and can cause fatal conditions by aspiration pneumonia and empyema (8), which is a serious complication that requires emergency surgical intervention.
This study aimed to investigate the occurrence of BPF in MIA in our department and discuss the causes based on the case, as well as compare the occurrence of BPF with open chest surgery and MIA during the same period. We present the following article in accordance with the STROBE reporting checklist (available at https://vats.amegroups.com/article/view/10.21037/vats-22-43/rc).
Methods
RATS lobectomy and U-VATS lobectomy were introduced in April 2016 and April 2019 in our department, respectively. Indications for MIA include stage I primary lung cancer, metastatic lung cancer, and benign diseases. Cases of undiagnosed tumors and benign diseases are not treated by RATS, and the decision to use uni-port or multi-port depends on the surgeon, but we have an educational program to become proficient in M-VATS before performing U-VATS.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Review Board for Clinical Studies at Osaka University (control number 10026-3) and individual consent for 2 patients of case presentation was obtained, but other patients for retrospective analysis was waived.
Patients
This study included 680 cases of pulmonary resection excluding wedge resection from December 2017 to August 2022. This study investigated the causative diseases classified as primary lung cancer, metastatic lung cancer, or benign diseases, such as infection or congenital cysts, and the surgical technique, approach. Patients were divided into two groups according to the presence or absence of BPF, and patient backgrounds were compared; multivariate analysis was performed to try to identify risk factors., and presence or absence of BPF. Patients resulted in BPF were examined for comorbidity, details of the operation and postoperative management, and time from surgery to BPF, and treatment.
Surgical procedures
M-VATS was conducted using two windows and one port, with the windows located on the anterior axillary line of the fourth intercostal space, the posterior axillary line of the sixth-seventh intercostal spaces, and the midaxillary line of the seventh intercostal space. U-VATS was performed through a small 3.5–4-cm incision on the anterior-mid axillary line of the fifth intercostal space on both sides. RATS was first performed with the DaVinci Surgical System Si® (Intuitive Surgical Inc., Sunnyvale, CA, USA), but is now conducted with the DaVinci Surgical System Xi® with five working ports. Four DaVinci ports are placed in the eighth or ninth intercostal space and the others on the anterior axillary line of the fourth intercostal space for assistance. The bronchus is often cut using a stapler. Staplers include Powered Echelon FLEX® (Johnson & Johnson Inc., New Brunswick, NJ, USA), SIGNIA® or ENDO GIA® with Tri-staple® (Medtronic Inc., Minneapolis, MN, USA), and Sureform® (Intuitive Surgical). The stapler and staple height depended on the surgeon’s preference. Cases with bronchus closure using a suture without a stapler in open chest surgery were included. The direction of bronchial dissection was not determined by Sweet’s or Overholt’s method. The bronchial stump cover was added by polyglycolic acid sheets, free/pedicled subcutaneous or mediastinal fat, and intercostal muscle, and the application of fibrin glue to prevent BPF in patients at high risk for delayed wound healing, such as those with diabetes, the elderly, and with steroid administration. Intercostal muscle flaps were not used in MIA but only fat pad and polyglycolic acid sheets. If BPF occurs, it is not often to monitor by only chest tube drainage and bronchoscopic treatment. Fenestration is the first procedure to be considered in principle. Then, when the cavity becomes clean by gauze drainage, then filling the cavity using a muscle and/or omentum is performed.
Statistical analysis
The two groups were compared using Fischer’s exact test, with a chi-squared test for categorical variables. Multivariate analysis was done using logistic regression analysis. Probability values of <0.05 were considered significant. Statistical comparisons were not performed in MIA because of the small number of BPF cases. BPF and non-BPF groups were compared for total pneumonectomy. All analyses were performed using the JMP15.0.1 statistical software package (SAS Institute Inc., Cary, NC, USA).
Results
This study enrolled 680 cases, including 544 lobectomies and bilobectomies, 124 segmentectomies, and 12 pneumonectomies. The most common approaches were M-VATS in 341 cases, followed by thoracotomy in 218 cases, U-VATS in 77 cases, and RATS in 42 cases. Lung cancer was the most reported disease. BPF occurred in 8 cases, but only 2 were in the MIA group. All 8 patients with BPF had covered margins and fibrin glue. Four cases had late onset, as well as both MIA cases. All 12 pneumonectomy cases were performed coverage the bronchial stumps with pedicled autologous tissue.
The BPF incidence by resection was 0.18% for lobectomy, 0.8% for segmentectomy, and 41.7% for pneumonectomy, whereas 0.29% for M-VATS, 0% for U-VATS, and 2.3% for RATS by surgical approach. The most reported diseases were benign at 4.5%, followed by metastatic lung cancer at 1.6% and lung cancer at 0.87%. BPF were more common in patients with open thoracotomy (P=0.0088), pneumonectomy (P<0.0001), or benign disease (P=0.029) (Table 1), while pneumonectomy was a significant factor in multivariate analysis (P<0.001) (Table 2). The incidence of BPF in the 189 patients in the open thoracotomy group, which excluded total pneumonectomy, was 1.1%, slightly higher than in the MIA group, but there was not statistically significant.
Table 1
| Variables | BPF (n=8) | No BPF (n=672) | P value |
|---|---|---|---|
| Sex | 0.0762 | ||
| Male | 7 | 378 | |
| Female | 1 | 294 | |
| Age (years) | 65.2±6.2 | 67.2±12.3 | 0.650 |
| Side | 0.679 | ||
| Right | 4 | 385 | |
| Left | 4 | 287 | |
| Approach | 0.0088 | ||
| Open | 6 | 212 | |
| MIA | 2 | 460 | |
| M-VATS | 1 | 341 | |
| RATS | 1 | 42 | |
| Uniportal VATS | 0 | 77 | |
| Procedure | <0.0001 | ||
| Pneumonectomy | 5 | 7 | |
| Not pneumonectomy | 3 | 665 | |
| Bilobectomy | 0 | 10 | |
| Lobectomy | 2 | 532 | |
| Segmentectomy | 1 | 123 | |
| Disease | 0.029 | ||
| Malignant | 6 | 631 | |
| Primary lung cancer | 6 | 568 | |
| Metastasis | 0 | 63 | |
| Benign | 2 | 41 | |
| Inflammatory | 2 | 14 | |
| No inflammatory | 0 | 27 |
Data are shown as number or mean ± standard deviation. BPF, broncho-pleural fistula; MIA, minimally invasive approach; M-VATS, multi-port video-assisted thoracoscopic surgery; RATS, robot assisted thoracic surgery.
Table 2
| Variables | Odds ratio (95% CI) | P value |
|---|---|---|
| Approach | ||
| Open | 1.02 (0.09–11.5) | 0.985 |
| MIA | Reference | – |
| Procedure | ||
| Pneumonectomy | 145.9 (14.5–1,469) | <0.001 |
| Not pneumonectomy | Reference | – |
| Disease | ||
| Benign | 3.98 (0.47–33.9) | 0.207 |
| Malignant | Reference | – |
MIA, minimally invasive approach.
BPF due to MIA occurred in 2 patients with RATS lobectomy for primary lung cancer and M-VATS right S6 segmentectomy for metastatic lung cancer. Six cases resulted in BPF in the thoracotomy group. Five used circulation support devices, such as artificial cardiopulmonary bypass or extracorporeal membrane oxygenation. Four patients required long-term mechanical ventilation for >1 week. Seven cases of BPF were covered with autologous tissue. Fenestration was performed in 7, but the closure the cavity was done in only 1 case (Table 3). The BPF group was significantly more likely to use mechanical ventilation and circulation support devices in pneumonectomy (P=0.045 each), but without differences in age, gender, disease, diabetes mellitus, or presence of immunosuppressive drugs, including steroids, and covering of stumps.
Table 3
| Case | Age (years) | Sex | Primary disease | Approach | Procedure | Comorbidity | Period from operation, month | Method | Covering of stump | Treatment of BPF |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | M | LC | RATS | LUL | COPD | 12 | Stapling | Free fat | Completion pneumonectomy |
| 2 | 71 | M | LC | M-VATS | Right S6 segmentectomy | DM | 2 | Stapling | PGA sheet | Fenestration, muscle filling |
| 3 | 59 | M | Asp | Open | RUML | CS, MV, M | 1 | Stapling | Pedicled fat | Fenestration |
| 4 | 59 | F | Sarcoma* | Open | Left pneumonectomy | CS, MV | 1 | Stapling | Pedicled fat | Fenestration |
| 5 | 71 | M | LC | Open | Right pneumonectomy | CS, MV | 1 | Suturing | Pedicled fat | Fenestration |
| 6 | 68 | M | LC | Open | Right pneumonectomy | M | 1.5 | Stapling | Intercostalis | Fenestration |
| 7 | 63 | M | LC | Open | Right pneumonectomy | CS | 1.5 | Suturing | Intercostalis | Fenestration |
| 8 | 73 | M | Asp | Open | Right pneumonectomy | CS, MV, M | 1 | Suturing | Intercostalis | Fenestration |
*, angiosarcoma of pulmonary artery. LC, lung cancer; Asp, chronic pulmonary aspergillosis; RATS, robot-assisted thoracoscopic surgery; M-VATS, multi-port video-assisted thoracoscopic surgery; LUL, left upper lobectomy; RUML, right upper and middle bilobectomy; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; CS, circulation support including cardiopulmonary bypass or extracorporeal membrane oxygenation; MV, mechanical ventilation; M, malnutrition; PGA, polyglycolic acid; BPF, broncho-pleural fistula.
BFF occurred in none of lower lobectomy, in which of the 97 cases were performed right lower lobectomy, which were done by thoracotomy in 32 and MIA in 65. In this group, 86 cases (88.5%) were performed covering of bronchial stump. Free fat pad was used in 24 cases, pedicled fat pad in 3 cases and PGA sheets in 27 cases on MIA.
Discussion
BPF occurs in a certain percentage of patients undergoing pulmonary resection and often requires surgical intervention because of rare spontaneous healing. Its occurrence is more common in right-sided surgery, especially after right pneumonectomy, as well as lower lobectomy and middle and lower bilobectomy (9). The incidence rate of BPF in pneumonectomy is reported as 1.5–8%, with a high fatality rate of 13.4–67% (10). The next most common type of resection is bilobectomy in 2.2–2.5%, and even less frequently by lobectomy in 0.5–3% (11,12). The frequency is even lower after segmentectomy at 0–0.3% (13).
BFF occurs more frequently postoperatively in benign diseases, such as non-tuberculous mycobacteriosis, tuberculosis, and other infectious diseases, with an incidence rate of 4.2–27% (14,15), especially high in right pneumonectomy (16). Risk factors besides infection were reported, including post-chemoradiotherapy, diabetes mellitus, steroid administration, etc. (17). Considerably, BPF is caused by prolonged bronchial inflammation and bronchial segment ischemia, leading to microvascular injury and decreased blood supply from the surrounding tissue.
BPF is few after segmentectomy, but some studies reported left upper segmentectomy M-VATS (13) and right basal segmentectomy M-VATS (18), in addition to S6 segmentectomy M-VATS in our case. These reports indicate a BPF incidence on VATS segmentectomy of 0.2–2.2% (13,18,19). The cause is reported to be silicosis of lymph nodes that hardly adhered to the bronchus or decreased blood flow from surrounding tissue compared to lobectomy.
The BPF incidence in RATS is 0.4–0.6% (20,21) and the occurrence is twice as high as in VATS, but the rate is not statistically significant, and the risk is similar to that of VATS (21).
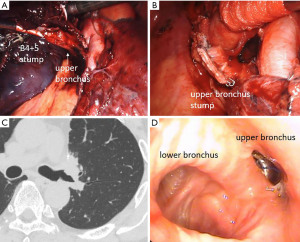
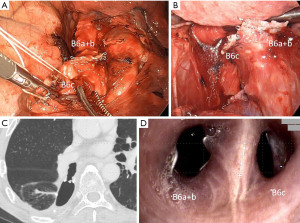
The BPF incidence in MIA in our cases was similar to a previous report. The common feature of our two BPF cases in MIA is that they required two stapling procedures, but the first case was centrally re-stapled and the second was dissected at the subsegmental bronchus level, which is different. Case 1 was young and had no specific comorbidity other than chronic obstructive pulmonary disease (COPD) (Figure 1). Thus, the risk of BPF was probably low, considerably because the tension of the stump worked strongly since the re-resection was centrally performed in an area where the bronchus became more rigid and larger. The diameter of the bronchus becomes larger, thereby requiring more pressure to close the tissue and the tension of the stump will become stronger because the flexibility of the bronchus depends on its thickness and length and each bronchus has a different diameter and length according to its ventilatory area (13). Additionally, the first stapling fixed the orientation of the peripheral bronchus and the twist of the bronchus was considered to cause the tension of the second stapling. Case 2 was a high-risk case because of diabetes mellitus and interstitial pneumonia, but we did not cover the end of the bronchus with self-tissue because it was a segmentectomy (Figure 2). Further, the two stapling sites, diabetes mellitus, and prolonged inflammation due to postoperative pneumonia may have contributed to the decreased blood flow at the bronchial stumps. It is considered an additional procedure, such as bronchial covering, may be desirable in patients at high risk for delayed wound healing even in segmentectomy.
The effectiveness of bronchial stump coverage has been reported in a randomized trial with intercostal muscles (22). Fat is often used (23) which is said to promote angiogenesis and tissue repair of the bronchial stump (24). Besides that, many other tissues have been reported to use it (25). In this series, BPF did not occur in lower lobectomy, and aggressive autologous tissue coverage or PGA sheet with fibrin glue may be effective in high-risk patients, even with MIA. Although PGA sheet coverage was used in segmentectomy and low-risk cases, it may be inferior to autologous tissue coverage because BPF occurred in one case. However, we believe this simple method is useful especially in cases such as lower lobectomy in low-risk patients, the effectiveness of the product has not been proven in this series due to the selection bias of the attending surgeon. All pneumonectomy cases were performed coverage with pedicled autologous tissue but developed BPF at a high rate. Seven cases of BPF who failed to prevent BPFs were covered with autologous tissue. But symptoms caused by BPF-like aspiration pneumonia or empyema were not severe in all cases. Hence, we could adequately prepare and consider the next plan for treatment of BPF. Additionally, no BPF-related deaths occurred. The autologous tissue covering cannot completely prevent BPF (26) and it is controversial whether all patients get advantages from coverage of bronchial stump, and which is the ideal tissue to use. But it may be effective in preventing the symptom caused by large, life-threatening fistulas.
However, BPF occurred at a high rate in thoracotomy, it is considered the reason there were many pneumonectomy cases who received mechanical ventilation under positive pressure and/or a cardiopulmonary support device. The number of cases is statistically significant although it was small, and the use of an assistive circulatory device and ventilatory management loads on a bronchial stump and such patients are considered to have a high-risk of BPF, but we consider there is no difference in the incidence of BPF between approaches.
Fenestration is the standard in principle in our institution if BPF occurs, but there have been reports of conservative improvement with fibrin glue (27), and closure with a stent (28). Although we have little experience with such conservative treatment, it is possible that success may be expected in the appropriate cases. As for case 1, a completion pneumonectomy was done due to the possibility of pulmonary artery bronchopleural fistula because of the presence of whole blood sputum. Hemoptysis caused by BPF has also been reported (29), which is a complication with a high mortality rate.
The limitation of this study is as follows. Is its retrospective design with a selection bias by the surgeon when he/she decided which approach or method of coverage was to be selected. We cannot compare the risk between open thoracotomy and MIA in pneumonectomy or right middle and lower lobectomy, which are generally associated with a high BPF incidence, because these two procedures were not performed in MIA in our hospital. In addition, the inability of statistical comparisons because of the small number of cases. Especially, the statistical analysis is insufficient because the comparison after correction by propensity score matching could not available. Additionally, this study included cases with a short observation period; thus, a follow-up with the occurrence of late BPF is necessary, as well as the accumulation of more experiences in the future.
Conclusions
The most at risk of BPF was pneumonectomy and it was considered there was no difference in the frequency of BPF between MIA and open thoracotomy. However, the incidence rate of BPF was low in MIA, it is possible that BPF may occur regardless of approach and procedure. It was thought that particular attention should be paid to multiple stapling procedures regarding the bronchus.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://vats.amegroups.com/article/view/10.21037/vats-22-43/rc
Data Sharing Statement: Available at https://vats.amegroups.com/article/view/10.21037/vats-22-43/dss
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-22-43/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-22-43/coif). NO serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from April 2022 to March 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Review Board for Clinical Studies at Osaka University (control number 10026-3) and individual consent for 2 patients of case presentation was obtained, but other patients for retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Ye X, Xie L, Chen G, et al. Robotic thoracic surgery versus video-assisted thoracic surgery for lung cancer: a meta-analysis. Interact Cardiovasc Thorac Surg 2015;21:409-14. [Crossref] [PubMed]
- Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg 2016;5:76-84. [Crossref] [PubMed]
- Cai YX, Fu XN, Xu QZ, et al. Thoracoscopic lobectomy versus open lobectomy in stage I non-small cell lung cancer: a meta-analysis. PLoS One 2013;8:e82366. [Crossref] [PubMed]
- Spiezia L, Liew A, Campello E, et al. Thrombotic risk following video-assisted thoracoscopic surgery versus open thoracotomy: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg 2020;30:573-81. [Crossref] [PubMed]
- Zhang R, Ferguson MK. Video-Assisted versus Open Lobectomy in Patients with Compromised Lung Function: A Literature Review and Meta-Analysis. PLoS One 2015;10:e0124512. [Crossref] [PubMed]
- Asamura H, Naruke T, Tsuchiya R, et al. Bronchopleural fistulas associated with lung cancer operations. Univariate and multivariate analysis of risk factors, management, and outcome. J Thorac Cardiovasc Surg 1992;104:1456-64. [Crossref] [PubMed]
- Darling GE, Abdurahman A, Yi QL, et al. Risk of a right pneumonectomy: role of bronchopleural fistula. Ann Thorac Surg 2005;79:433-7. [Crossref] [PubMed]
- Pforr A, Pagès PB, Baste JM, et al. A Predictive Score for Bronchopleural Fistula Established Using the French Database Epithor. Ann Thorac Surg 2016;101:287-93. [Crossref] [PubMed]
- Sonobe M, Nakagawa M, Ichinose M, et al. Analysis of risk factors in bronchopleural fistula after pulmonary resection for primary lung cancer. Eur J Cardiothorac Surg 2000;18:519-23. [Crossref] [PubMed]
- Sirbu H, Busch T, Aleksic I, et al. Bronchopleural fistula in the surgery of non-small cell lung cancer: incidence, risk factors, and management. Ann Thorac Cardiovasc Surg 2001;7:330-6. [PubMed]
- Oizumi H, Kato H, Endoh M, et al. Management of Bronchial Stumps in Anatomic Lung Segmentectomy. Ann Thorac Surg 2016;101:2120-4. [Crossref] [PubMed]
- Mitchell JD, Bishop A, Cafaro A, et al. Anatomic lung resection for nontuberculous mycobacterial disease. Ann Thorac Surg 2008;85:1887-92; discussion 1892-3. [Crossref] [PubMed]
- Shiraishi Y, Nakajima Y, Katsuragi N, et al. Pneumonectomy for nontuberculous mycobacterial infections. Ann Thorac Surg 2004;78:399-403. [Crossref] [PubMed]
- Conlan AA, Kopec SE. Indications for pneumonectomy. Pneumonectomy for benign disease. Chest Surg Clin N Am 1999;9:311-26. [PubMed]
- Salik I, Vashisht R, Abramowicz AE. Bronchopleural Fistula. Treasure Island (FL): StatPearls Publishing; 2022.
- Sang Y, Chen Y, Zhang Z, et al. Multidisciplinary team approach on a case of bronchopleural fistula after video-assisted thoracoscopic segmentectomy: a case report. Transl Cancer Res 2020;9:4036-42. [Crossref] [PubMed]
- Ueno T, Maki Y, Sugimoto R, et al. Therapeutic Outcomes of 15 Postoperative Bronchopleural Fistulas Including Seven Endoscopic Interventions. Acta Med Okayama 2019;73:325-31. [PubMed]
- Cao C, Louie BE, Melfi F, et al. Outcomes of major complications after robotic anatomic pulmonary resection. J Thorac Cardiovasc Surg 2020;159:681-6. [Crossref] [PubMed]
- Louie BE, Wilson JL, Kim S, et al. Comparison of Video-Assisted Thoracoscopic Surgery and Robotic Approaches for Clinical Stage I and Stage II Non-Small Cell Lung Cancer Using The Society of Thoracic Surgeons Database. Ann Thorac Surg 2016;102:917-24. [Crossref] [PubMed]
- Sfyridis PG, Kapetanakis EI, Baltayiannis NE, et al. Bronchial stump buttressing with an intercostal muscle flap in diabetic patients. Ann Thorac Surg 2007;84:967-71. [Crossref] [PubMed]
- Brewer LA 3rd, King EL, Lilly LJ, et al. Bronchial closure in pulmonary resection: a clinical and experimental study using a pedicled pericardial fat graft reinforcement. J Thorac Surg 1953;26:507-32. [Crossref] [PubMed]
- Ichinose Y, Asoh H, Yano T, et al. Use of a pericardial fat pad flap for preventing bronchopleural fistula: an experimental study focusing on the angiogenesis and cytokine production of the fat pad. Surg Today 1995;25:811-5. [Crossref] [PubMed]
- Anderson TM, Miller JI Jr. Use of pleura, azygos vein, pericardium, and muscle flaps in tracheobronchial surgery. Ann Thorac Surg 1995;60:729-33. [Crossref] [PubMed]
- Skrzypczak P, Roszak M, Kasprzyk M, et al. The technique of stump closure has no impact on post-pneumonectomy bronchopleural fistula in the non-small cell lung cancer-a cross-sectional study. J Thorac Dis 2022;14:3343-51. [Crossref] [PubMed]
- Cardillo G, Carbone L, Carleo F, et al. The Rationale for Treatment of Postresectional Bronchopleural Fistula: Analysis of 52 Patients. Ann Thorac Surg 2015;100:251-7. [Crossref] [PubMed]
- Han X, Yin M, Li L, et al. Customized airway stenting for bronchopleural fistula after pulmonary resection by interventional technique: single-center study of 148 consecutive patients. Surg Endosc 2018;32:4116-24. [Crossref] [PubMed]
- Brik A, Salem AM, Shoukry A, et al. Surgery for hemoptysis in various pulmonary tuberculous lesions: a prospective study. Interact Cardiovasc Thorac Surg 2011;13:276-9. [Crossref] [PubMed]
Cite this article as: Ose N, Funaki S, Fukui E, Kanou T, Kimura T, Shintani Y. Experiences of broncho-pleural fistula after pulmonary resection in minimally invasive approach and open thoracotomy. Video-assist Thorac Surg 2023;8:1.


