
Simulation and navigation techniques in segmentectomy for lung cancer
Introduction
Lung cancer is the leading cause of death worldwide, and lobectomy has been the standard surgical procedure for lung cancer since the results of a historical prospective clinical trial by the Lung Cancer Study Group (LCSG) in 1995 (1). However, the diagnostic imaging technology for lung cancer in the 1980s, which was used in this study conducted by the LCSG, is poor compared with modern technology, especially in terms of the resolution and accuracy of computed tomography (CT). With the subsequent worldwide spread of high-resolution (HR) CT, studies of well-differentiated lung adenocarcinoma exhibiting ground-glass opacity (GGO) on CT (2,3), studies of the relationship between the ratio of consolidation to the total tumor diameter (consolidation to tumor ratio; C/T ratio) in CT images and prognosis of lung cancer (4,5), and a single center study of sublobar resection for lung cancer as an intentional surgery (6,7), were reported and evidence has been accumulated. Due to such changes in the field of lung cancer, a large-scale prospective clinical trial was planned in Japan (JCOG0802/WJOG4607L) which matches the current level of lung cancer treatment. The results showed that intentional segmentectomy for small-sized lung cancer significantly improved overall survival (OS) compared with standard lobectomy (8).
From a technical perspective, new research on lung segmental anatomy has been reported since the 2010s with the advent of three-dimensional (3D) CT analysis software using HRCT (9,10). Additionally, with advances in thoracoscopic surgery, video-assisted thoracoscopic surgery (VATS) segmentectomy using preoperative 3D simulation has been reported (11-13), and is expected to become widespread. In this review article, we introduce the evolution of 3D-CT simulation and navigation with the demand for segmentectomy for lung cancer, and the latest 3D-CT simulation software specialized for lung segmentectomy that we use at Shinshu University. Furthermore, we describe other new 3D technologies such as virtual reality (VR) technology in the area of thoracic surgery.
Development of lung cancer imaging by computed tomography
The historical prospective clinical trial reported by the LCSG in 1995 included radiographically detected lung cancers ≤3 cm. Patient eligibility was described as “All patients had suspected lung cancer discovered on chest roentgenogram” (1). Since then, diagnostic imaging technology has improved drastically. Due to advances in CT equipment, surgical resections have increased for small-sized lung cancers and low-grade lung cancer with GGO that are undetected on chest radiographs.
In 2003 , Ohde et al. have reported a study that investigated HRCT findings of lung cancer ≤3 cm in 101 lesions from 98 patients (4). They showed that non-invasive disease (tumor without lymph node involvement or vessel invasion) could be determined when the ratio of Cdmax (greatest diameter of consolidation found on all CT cuts) and Td (greatest diameter of tumor found on all CT cuts) was <0.5 (4). Based on this concept of consolidation to tumor ratio (C/T ratio), the results of the Japan Clinical Oncology Group (JCOG) 0201 study were reported in 2011. Suzuki et al. have found that a lung adenocarcinoma ≤2.0 cm with a C/T ratio ≤0.25 was defined as the cutoff for radiological non-invasive lung cancer that predicts pathological non-invasive lung cancer (5). In this study, the provisional pathological definition of non-invasive lung cancer is lung adenocarcinoma without nodal involvement, vascular invasion, or lymphatic invasion. They concluded that wide-wedge resection, as a limited resection, could be adopted because these tumors have a limited potential for nodal involvement or lymphatic/vascular invasion (5).
These results identified which lung cancer types could undergo limited resection, based on preoperative CT findings. However, even if the lesion meets these conditions, segmentectomy should be selected when a wide-wedge resection is not feasible because the lesion is deeply located in the lung parenchyma.
Impact of segmentectomy for lung cancer
Since the report by LCSG (1), lobectomy has been the standard surgical procedure for lung cancer for over two decades. However, their study was based on medical standards from the 1980s. Since then, subsequent improvements in diagnostic imaging technology have resulted in small-sized lung cancers being detected on HRCT. Accordingly, results of intentional sublobar resection for stage I lung cancer was retrospectively analyzed, and several favorable results were reported. Kodama et al. have analyzed 46 patients who underwent intentional segmentectomy, and Koike et al. have evaluated 74 patients undergoing sublobar resection, including 14 wedge resections, and compared them with standard lobectomy and reported similar prognosis (6,7). Okada et al. have assessed 1,272 non-small cell lung cancer patients at a single institution and concluded that segmentectomy may be acceptable for patients with a tumor ≤2 cm in diameter without nodal involvement (14). Furthermore, their group reported a non-randomized trial conducted at 3 centers including 305 cases of segmentectomy without nodal involvement, lesions ≤2 cm, and lobectomy tolerance. Analysis showed that the recurrence rate and prognosis were not inferior to 262 patients who underwent lobectomy (15). Harada et al. have further evaluated the effect of segmentectomy on respiratory function and reported the functional advantage of segmentectomy over lobectomy. They found that a positive and significant correlation between the number of resected segments versus loss of forced vital capacity, loss of forced expiratory volume in 1 second at 2 and 6 months postoperatively, and the postoperative reduction of forced expiratory volume in 1 second was significantly less in the segmentectomy group (16).
In Japan, a prospective randomized study of segmentectomy versus lobectomy (JCOG0802/WJOG4607L) was started in 2008 (17), and the results were recently published (8). The benefit in respiratory function for the segmentectomy group was only 3.5% in the differences in proportion of median forced expiratory volume in 1 second at 12 months from surgery compared to lobectomy group, which was lower than the initial prediction. Surprisingly, the OS after segmentectomy was significantly better than that post lobectomy. However, the local relapse after segmentectomy was approximately twice as high (10.4% versus 5.4%), which underlines the importance of securing a sufficient surgical margin during segmentectomy.
A prospective phase 3 trial similar to JCOG0802//WJOG4607L was conducted by international group of Australia, Canada, and the USA (CALGB/Alliance 140503). Clinically T1aN0 (TNM7th, ≤2 cm) non-small-cell lung cancer patients were recruited for this study, and the primary endpoint is disease-free survival. This trial differs from JCOG trial in that it includes a wedge resection, and the equivalence of sublobar resection to lobectomy was presented at the 2022 World Congress of Lung Cancer, however, the final report is awaited.
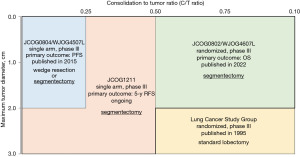
Figure 1 shows the criteria for selecting surgical procedures in patients with stage I lung cancer, based on Japanese clinical trials (8,18,19). As indicated by these published results and ongoing clinical trials, the importance and demand for segmentectomy may increase in the future, hence, all stage I lung cancer patients were candidate for lobectomy, previously. Furthermore, this indicates that the preoperative simulation based on 3D-CT will be an equally crucial factor as the surgical technique.
Segmental anatomy by 3D-CT of the lung
The development of HRCT has increased the detection of small-sized lung cancers and ground-glass nodules. Additionally, with the development of 3D-CT technology, a 3D construction of pulmonary vessels, especially pulmonary arteries (20), research on segmental anatomy including bronchi and pulmonary arteries and veins, and research on segmentectomy using these anatomical data have advanced (11,12).
Shimizu and colleagues conducted several segmental anatomical studies using 3D-CT and reported bronchovascular pattern variations of the right upper lobe, focusing on the pulmonary veins (9,10), and the variation patterns of the right middle and lower lobes (21). Additionally, they reported various new surgical procedures using 3D-CT such as the medial-basal segmentectomy (S7), subsuperior segmentectomy (S*), right middle lobe segmentectomy, medial-basal segment (S7)-sparing right basal segmentectomy, and superior lingular S4 segmentectomy (22-26).
Furthermore, Shimizu et al. have developed a simplified anatomical model for segmentectomy of the right upper lobe, focusing on the pulmonary vein variations (10), and reported the surgical outcomes of a model-based approach to the intersegmental vein (27). These studies focused on the relationship between bronchi and pulmonary veins because the branching pattern of veins is highly helpful for preoperative simulation and intraoperative navigation in right upper segmentectomy. Table 1 summarizes the reports of 3D-CT simulation for segmentectomy or anatomical lung resection in clinical practice.
Table 1
| Author | Number of cases | Procedure | Approach | Feature | 3D software | Year | Journal | References |
|---|---|---|---|---|---|---|---|---|
| Watanabe | 14 | Anatomic resection | Thoracotomy | 3DCTA; pulmonary artery | Somatom Plus 4-VZ; Siemens Medical System, Germany | 2003 | Ann Thorac Surg | (20) |
| Oizumi | NA | Segmentectomy | Thoracoscopic | 3DCTA; pulmonary artery and vein | 64-channel multi-detector CT | 2010 | Ann Thorac Surg | (11) |
| Shimizu | 42 | Segmentectomy | 27 hybrid VATS; 15 totally thoracoscopic |
3DCTAB; pulmonary artery/vein and bronchi | Ziostation, Ziosoft, Japan | 2012 | Interact Cardiovasc Thorac Surg | (12) |
| Nakazawa | 34 | RUL segmentectomy | Thoracoscopic | 3DCTAB; pulmonary artery/vein and bronchi | Ziostation, Ziosoft, Japan | 2020 | JTCVS Tech | (27) |
3D-CT, three-dimensional computed tomography; NA, not applicable; RUL, right upper lobe, VATS, video-assisted thoracoscopic surgery, 3DCTA, three-dimensional computed tomography angiography, 3DCTAB, three-dimensional computed tomography angiography and bronchography.
Simulation and navigation techniques in thoracoscopic segmentectomy
Segmentectomy is a challenging surgical procedure attributed to the anatomical complexity of the lung. In segmentectomy, it is critical to perform preoperative simulation while ensuring an appropriate surgical margin based on the tumor size, tumor localization, and radiological findings such as solid or ground-glass nodules. Therefore, precise 3D-CT simulations are useful.
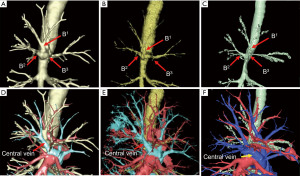
Recently, volume-rendering reconstruction software dedicated to lung segmentectomy was approved in Japan (REVORAS, 2020, Ziosoft, Inc., Tokyo, Japan). This software has new, advantageous functions with surgeon-oriented features as follows: semi-automated segmentectomy planning based on the location of the tumor and the dividing level of the bronchial or arterial branches (surgeons can determine any dividing level of the branches) and automated measurement of the margin distance. Specific 3D views for segmentectomy are useful in understanding the divided or preserved hilar structures and the shape of the lung parenchyma to be resected with the intersegmental plane (28). Although thin-slice enhanced CT is preferred for creating high-quality simulation images, even images created by plain CT are generally acceptable for clinical use. Another feature of REVORAS is that tracing the outer rim of the bronchi makes visualization clearer compared with the previous model Ziostation2 (2010, Ziosoft, Inc.). Figure 2 shows an image comparison between REVORAS, Ziostation2, and another widely used 3D software, Synapse Vincent (Ver.5.4, 2017, Fujifilm, Tokyo, Japan). The bronchial branches were more clearly and more distally recognized in REVORAS as compared with Ziostation2 and Synapse Vincent.
At Shinshu University Hospital, we adopted custom-made precision segmentectomy with preoperative simulation and intraoperative navigation using REVORAS in daily clinical practice. This includes both thoracotomy or thoracoscopic and VATS and robot-assisted thoracoscopic surgery (RATS) segmentectomy. For all patients undergoing segmentectomy, we create 3D-CT images to plan segmentectomy. Based on the planning with expected volume loss and margin distance, we determine which segment(s) and/or subsegment(s) should be removed and which segmental/subsegmental branches should be divided or preserved with adequate margins (28). The actual time required for deliberated simulation of segmentectomy for each case is about 10–20 minutes. Figure 3 shows an actual 3D simulation by REVORAS and an intraoperative view of VATS left S3b subsegmentectomy for a patient with a 4-mm metastatic lung tumor. In this case, the automatically simulated margin distance was 4.5 mm; hence, subsegmentectomy was performed by dissecting the V3c and extended into S3c and the pathological margin was negative.
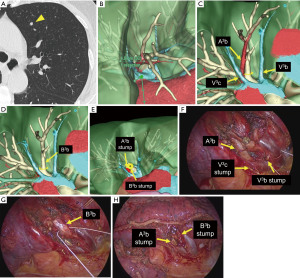
Preoperative simulation and intraoperative navigation by displaying the resected bronchi and pulmonary vessels using REVORAS can facilitate segmentectomy or complex subsegmentectomy with an appropriate dissection of the pulmonary vessels, bronchi, and lung parenchyma, which leads to an appropriate surgical margin.
A limitation of various 3D simulation software currently in use is that it does not reflect the collapsed lung during surgery because the simulation was performed using preoperative CT by fully inspirated state that is similar to a fully ventilated lung. Another limitation is the cost of the software. Commercially available 3D simulation software such as REVORAS, Ziostation, and Synapse Vincent are considerably expensive, and each institute must install them to use.
Other new 3D technologies in thoracic surgery
VR devices and software have become popular and are being tested for application in the field of thoracic surgery (29). Ujiie et al. have reported preoperative planning and simulation using VR technology by 3D polygon data derived from preoperative CT data and head-mount displays and applied their initial experience of lingula-sparing left upper segmentectomy (30). Li et al. have applied augmented reality (AR) and 3D-print models to 55 thoracoscopic lung segmentectomy or subsegmentectomy that revealed favorable outcomes such as shorter operating time, less intraoperative blood loss, and shorter length of hospital stay (31).
VR technology has further been applied to VATS training in the field of thoracic surgery. Jensen et al. have reported a simulation-based test of VATS lobectomy of a right upper lobe using a VR simulator and established valid evidence for a VR simulator test (32,33). Furthermore, Haidari et al. have described five VATS lobectomy simulations using a VR simulator (34,35).
Arjomandi Rad et al. have reviewed extended reality (XR), encompassing both VR and AR, and described three areas: (I) the application of XR in thoracic surgery training; (II) preoperative planning of thoracic procedures; and (III) intraoperative assistance from articles that explored the use of VR and/or AR (36). They emphasized the benefits of XR for surgical training, particularly in junior trainees, by allowing them to operate in a realistic, but ethically risk-free setting, as well as enhancing preoperative planning and intraoperative guidance (36).
Yoon et al. have reported another clinical application of 3D technology other than preoperative simulation or training. They improved the quality of informed consent using personalized 3D-printed lung models for lung cancer patients. This method helped obtain preoperative informed consent and improved patient comprehension (37).
Tokuno et al. have reported an original software called resection process map (RPM) that can create dynamic virtual images (deformable images) that enable more accurate intraoperative simulation (38,39).
Several of these technologies are still under development, however, we expect further progress in this field.
Conclusions
In the field of thoracic surgery, the demand for segmentectomy, which requires more complex and precise planning and procedures, will inevitably increase. Rapidly advancing 3D-based simulations will enable more precise and accurate surgical planning, navigation, and surgical training. These are expected to contribute to safe surgery, which will lead to improved surgical outcomes.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Seshiru Nakazawa and Hitoshi Igai) for the series “Simulation and Navigation Techniques in VATS/RATS” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-22-42/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-22-42/coif). The series “Simulation and Navigation Techniques in VATS/RATS” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Ann Thorac Surg 1995;60:615-23. [Crossref] [PubMed]
- Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995;75:2844-52. [Crossref] [PubMed]
- Travis WD, Garg K, Franklin WA, et al. Evolving concepts in the pathology and computed tomography imaging of lung adenocarcinoma and bronchioloalveolar carcinoma. J Clin Oncol 2005;23:3279-87. [Crossref] [PubMed]
- Ohde Y, Nagai K, Yoshida J, et al. The proportion of consolidation to ground-glass opacity on high resolution CT is a good predictor for distinguishing the population of non-invasive peripheral adenocarcinoma. Lung Cancer 2003;42:303-10. [Crossref] [PubMed]
- Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011;6:751-6. [Crossref] [PubMed]
- Kodama K, Doi O, Higashiyama M, et al. Intentional limited resection for selected patients with T1 N0 M0 non-small-cell lung cancer: a single-institution study. J Thorac Cardiovasc Surg 1997;114:347-53. [Crossref] [PubMed]
- Koike T, Yamato Y, Yoshiya K, et al. Intentional limited pulmonary resection for peripheral T1 N0 M0 small-sized lung cancer. J Thorac Cardiovasc Surg 2003;125:924-8. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Nagashima T, Shimizu K, Ohtaki Y, et al. An analysis of variations in the bronchovascular pattern of the right upper lobe using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg 2015;63:354-60. [Crossref] [PubMed]
- Shimizu K, Nagashima T, Ohtaki Y, et al. Analysis of the variation pattern in right upper pulmonary veins and establishment of simplified vein models for anatomical segmentectomy. Gen Thorac Cardiovasc Surg 2016;64:604-11. [Crossref] [PubMed]
- Oizumi H, Endoh M, Takeda S, et al. Anatomical lung segmentectomy simulated by computed tomographic angiography. Ann Thorac Surg 2010;90:1382-3. [Crossref] [PubMed]
- Shimizu K, Nakano T, Kamiyoshihara M, et al. Segmentectomy guided by three-dimensional computed tomography angiography and bronchography. Interact Cardiovasc Thorac Surg 2012;15:194-6. [Crossref] [PubMed]
- Shimizu K, Nakazawa S, Nagashima T, et al. 3D-CT anatomy for VATS segmentectomy. J Vis Surg 2017;3:88. [Crossref] [PubMed]
- Okada M, Nishio W, Sakamoto T, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg 2005;129:87-93. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Harada H, Okada M, Sakamoto T, et al. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005;80:2041-5. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Suzuki K, Watanabe S, Mizusawa J, et al. Predictors of non-neoplastic lesions in lung tumours showing ground-glass opacity on thin-section computed tomography based on a multi-institutional prospective study. Interact Cardiovasc Thorac Surg 2015;21:218-23. [Crossref] [PubMed]
- Aokage K, Saji H, Suzuki K, et al. A non-randomized confirmatory trial of segmentectomy for clinical T1N0 lung cancer with dominant ground glass opacity based on thin-section computed tomography (JCOG1211). Gen Thorac Cardiovasc Surg 2017;65:267-72. [Crossref] [PubMed]
- Watanabe S, Arai K, Watanabe T, et al. Use of three-dimensional computed tomographic angiography of pulmonary vessels for lung resections. Ann Thorac Surg 2003;75:388-92; discussion 392. [Crossref] [PubMed]
- Nagashima T, Shimizu K, Ohtaki Y, et al. Analysis of variation in bronchovascular pattern of the right middle and lower lobes of the lung using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg 2017;65:343-9. [Crossref] [PubMed]
- Shimizu K, Nagashima T, Yajima T, et al. Thoracoscopic Medial-Basal Segment Segmentectomy. Ann Thorac Surg 2017;104:e403-6. [Crossref] [PubMed]
- Shimizu K, Mogi A, Yajima T, et al. Thoracoscopic Subsuperior Segment Segmentectomy. Ann Thorac Surg 2017;104:e407-10. [Crossref] [PubMed]
- Yajima T, Shimizu K, Mogi A, et al. Thoracoscopic right middle lobe segmentectomy. Gen Thorac Cardiovasc Surg 2019;67:344-7. [Crossref] [PubMed]
- Yajima T, Shimizu K, Mogi A, et al. Medial-basal segment (S(7))-sparing right basal segmentectomy. Gen Thorac Cardiovasc Surg 2020;68:306-9. [Crossref] [PubMed]
- Nakazawa S, Yajima T, Numajiri K, et al. Superior Lingular S(4) Segmentectomy. Ann Thorac Surg 2022;113:e141-4. [Crossref] [PubMed]
- Nakazawa S, Shimizu K, Kawatani N, et al. Right upper lobe segmentectomy guided by simplified anatomic models. JTCVS Tech 2020;4:288-97. [Crossref] [PubMed]
- Eguchi T, Sato T, Shimizu K. Technical Advances in Segmentectomy for Lung Cancer: A Minimally Invasive Strategy for Deep, Small, and Impalpable Tumors. Cancers (Basel) 2021;13:3137. [Crossref] [PubMed]
- Sadeghi AH, Maat APWM, Taverne YJHJ, et al. Virtual reality and artificial intelligence for 3-dimensional planning of lung segmentectomies. JTCVS Tech 2021;7:309-21. [Crossref] [PubMed]
- Ujiie H, Yamaguchi A, Gregor A, et al. Developing a virtual reality simulation system for preoperative planning of thoracoscopic thoracic surgery. J Thorac Dis 2021;13:778-83. [Crossref] [PubMed]
- Li C, Zheng B, Yu Q, et al. Augmented Reality and 3-Dimensional Printing Technologies for Guiding Complex Thoracoscopic Surgery. Ann Thorac Surg 2021;112:1624-31. [Crossref] [PubMed]
- Jensen K, Ringsted C, Hansen HJ, et al. Simulation-based training for thoracoscopic lobectomy: a randomized controlled trial: virtual-reality versus black-box simulation. Surg Endosc 2014;28:1821-9. [Crossref] [PubMed]
- Jensen K, Hansen HJ, Petersen RH, et al. Evaluating competency in video-assisted thoracoscopic surgery (VATS) lobectomy performance using a novel assessment tool and virtual reality simulation. Surg Endosc 2019;33:1465-73. [Crossref] [PubMed]
- Haidari TA, Bjerrum F, Hansen HJ, et al. Simulation-based VATS resection of the five lung lobes: a technical skills test. Surg Endosc 2022;36:1234-42. [Crossref] [PubMed]
- Haidari TA, Bjerrum F, Christensen TD, et al. Assessing VATS competence based on simulated lobectomies of all five lung lobes. Surg Endosc 2022;36:8067-75. [Crossref] [PubMed]
- Arjomandi Rad A, Vardanyan R, Thavarajasingam SG, et al. Extended, virtual and augmented reality in thoracic surgery: a systematic review. Interact Cardiovasc Thorac Surg 2022;34:201-11. [Crossref] [PubMed]
- Yoon SH, Park S, Kang CH, et al. Personalized 3D-printed model for informed consent for stage I lung cancer: a randomized pilot trial. Semin Thorac Cardiovasc Surg 2019;31:316-8. [Crossref] [PubMed]
- Tokuno J, Chen-Yoshikawa TF, Nakao M, et al. Resection Process Map: A novel dynamic simulation system for pulmonary resection. J Thorac Cardiovasc Surg 2020;159:1130-8. [Crossref] [PubMed]
- Tokuno J, Chen-Yoshikawa TF, Nakao M, et al. Creation of a video library for education and virtual simulation of anatomical lung resection. Interact Cardiovasc Thorac Surg 2022;34:808-13. [Crossref] [PubMed]
Cite this article as: Hamanaka K, Miura K, Eguchi T, Shimizu K. Simulation and navigation techniques in segmentectomy for lung cancer. Video-assist Thorac Surg 2023;8:4.


