
Role and evidence for targeted therapies in surgically resectable non-small cell lung cancer: a narrative review
Introduction
Cancer cells replicate at a faster rate than most normal tissues. Cytotoxic chemotherapy targets cell replication to halt cancer growth and reduce its volume, but responses are short-lived and the toxicity involved can be detrimental to the patient’s quality of life. The increasing knowledge in cancer biology over the last decades led the way to the introduction of novel targeted therapies in lung cancer, the tumour type with the highest mortality in the UK and the world (1). These new therapies target specific cancer processes, hence have the potential to be more effective and less toxic. They are designed to target cancer molecules or key players in communication pathways involved in hallmarks of cancer (2).
The therapeutic targets are receptors that are constitutively active or that are key for cancer survival. These targets include, on the one hand, mutated or translocated receptors that drive the growth of lung cancer. They are inhibited by small molecule drugs that bind to the intracellular tyrosine kinase domain and are called tyrosine kinase inhibitors (TKI) (3,4) (i.e., osimertinib, alectinib or entrectinib). Their drug names always end in -nib, the shorthand for “inhibitor”. On the other hand, there are antibodies such as nivolumab, atezolizumab or pembrolizumab, with the ending-mab for “monoclonal antibody”. These inhibit the extracellular domain of the receptor where the interaction between cancer cells and immune cells occurs. That interaction is called immune checkpoint, hence these antibodies are called immune checkpoint inhibitors (ICI) (5). In the advanced setting, novel targeted therapies can provide higher response rates, longer benefits and/or improved tolerability when compared with chemotherapy, although in a group of patients (i.e., never smokers or PD-L1 <50%) clinical benefit may be more evident combining immunotherapy with chemotherapy (6).
To optimize clinical efficacy in the early stage it is necessary to understand the effects of the drugs in the cancer tissue learning from the experience of these targeted treatments in advanced disease. For example, in mutation-driven lung cancers, TKIs may trigger an initial cytotoxic (cell-killing) effect with cancer volume reduction within the first few months. This is followed by a cytostatic phase, where growth is arrested. This is evident when the inhibitor is withheld due to toxicity or concurrent illness for long periods: the cancer will invariably grow again. When treatment is resumed, disease control is achieved if resistance has not developed. In these cancers, primary resistance—cancer growth when patients are first exposed to TKI—is rare, but acquired resistance, that is symptomatic cancer growth after an initial response had been observed, will invariably occur.
The rationale to combine TKIs with chemotherapy in the advanced setting is that by reducing tumour volume at the outset, cancer tissue may take longer to develop acquired resistance through the selection pressures inflicted by constant drug exposure (7-10). In the neoadjuvant setting, increasing the number of patients that achieve volume reduction may also help downstage the disease and increase cure rates.
Another important consideration is that these cancers are more common in younger patients and never-smokers (11), the prevalence of brain metastases is higher (12) and their response to single-agent immunotherapy is poor (13). Also, their post-surgical outcomes may be better than in wild type tumours (those without a driver mutation) (14). Mutation-driven lung cancers may represent a separate type of lung cancer altogether.
It is necessary to understand the meaning of each genetic anomaly individually. Whereas epidermal growth factor receptor (EGFR) mutations, or ALK translocations (perhaps also ROS1, NTRK or RET translocations) may fit the description above of a mutation-driven lung cancer, it is not the case in patients with, for example, KRASG12C mutations, which are more common in smokers and can even predict a good response to immunotherapy (15) or c-met exon 14 skipping mutation, also more common in smokers (16).
With ICI, PDL1 receptor expression may be a predictive factor of response [PD-L1 of 50% or more predicts good response and longer benefit to these inhibitors (17)], but overall disease control rates are not as high as with TKIs. Here the target is not a specific mutated receptor present in all cancer cells, but the dynamic interaction between the immune system and cancer tissue with heterogeneous expression of the receptor (18).
Novel targeted therapies have revolutionised lung cancer care in the advanced disease setting. Exploring their potential benefits in the early-stage disease, where cure is possible, was inevitable. As recent publications with targeted treatment therapy have emerged in early-stage lung cancer, there is a need to summarize the evidence for the multidisciplinary community. This review, in accordance with the narrative review reporting checklist, shall discuss the evidence available for both treatment types in the early stage setting and how these treatments may challenge the current diagnostic pathway, patient selection and current post-operative follow up guidelines. The author presents the following article in accordance with the Narrative Review reporting checklist (available at https://vats.amegroups.com/article/view/10.21037/vats-21-39/rc).
Methods
Different Medline and Google Scholar searches were made in August 2021 and again in December 2021 (see Table 1) with a combination of the words “early stage”, “lung cancer”, “neoadjuvant”, “adjuvant”, “EGFR”, “ALK”, “mutation-driven”, “oncogene addicted”, “immunotherapy” and “checkpoint inhibition”. This included studies between 1970 and 2021. Randomized studies were selected, and early-phase studies mentioned only when they were considered to inform patient management. Unreported phase III studies that are recruiting or have completed recruitment were searched via clinicaltrials.gov. They were mentioned only when the author considered their results may answer questions on the data discussed here or underlined areas where research is ongoing. Clinical trials with outdated selection criteria (i.e., EGFR inhibitor studies that did not select patients with EGFR mutations) were excluded. These searches were complemented with hand searches of the references of retrieved literature and updated clinical guidelines.
Table 1
| Items | Specification |
|---|---|
| Date of Search (specified to date, month and year) | August 15th, 2021 and December 22nd, 2021 |
| Databases and other sources searched | Medline and Google Scholar. These searches were complemented by manual searches from references of retrieved literature and updated clinical guidelines |
| Search terms used (including MeSH and free text search terms and filters) | Combination of the words “early stage”, “lung cancer”, “neoadjuvant”, “adjuvant”, “EGFR”, “ALK”, “mutation-driven”, “oncogene addicted”, “immunotherapy” and “checkpoint inhibition” |
| Timeframe | 1970–2021 |
| Inclusion and exclusion criteria (study type, language restrictions, etc.) | Randomized studies reported in the English language with selection criteria consistent with current practice were selected. Early-phase studies were referenced when the author considered they provided a relevant context to the larger study interpretation or when no large, randomized studies where available |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | The selection was conducted by the author, using his experience and feedback from previous public talks and meetings |
Results
The adjuvant setting
Systemic treatment after radical surgery offers the opportunity to increase the disease-free survival (DFS) or avoid recurrence altogether, at the expense of treating patients whose cancer would never recur. Clinical trial recruitment into the adjuvant setting allows, on the one hand, access to large amounts of tissue from the surgical specimen, and the possibility to identify the fittest patients by recruiting within a few weeks after surgery. On the other hand, recent major surgery may lower the threshold for subsequent treatment toxicity. More significantly, the lack of short-term outcomes that correlate with survival requires long follow up times, even though trial design limitations have relegated overall survival (OS) to a secondary objective in lung cancer subgroups with low incidence, such as mutation-driven cancers.
Up until recently, the standard of care in the adjuvant setting has been limited to cytotoxic chemotherapy (19). Two meta-analyses, one with fifty two studies (20) and a more recent one with five large studies (21), have shown an overall 5-year survival benefit of 5% using platinum-based chemotherapy. The benefit was most obvious in stage II and III disease, and was detrimental in patients with stage IA or WHO Performance Status (PS) of 2. The guidelines advise platinum combination should be considered in patients with tumours ≥4 cm (19). Following prospective evidence, treatment initiation is usually 6–8 weeks after surgery, but in retrospective studies the benefit of adjuvant chemotherapy can be identified up to 4 months after surgery (22). The small benefit that this treatment offers and individual patient circumstances (i.e., life priorities, post-operative fitness, and co-morbidities) means that not all patients that have surgery for ≥4 cm tumours have adjuvant chemotherapy.
TKI
Three years of adjuvant osimertinib in patients with common EGFR mutations (L858R or deletion 19) and at least 30 mm size tumours (T2aN0M0 or stage IB as per TNM version 7) is currently considered standard of care following the interim results published for the ADAURA study (23) (see Figure 1). This trial selected 682 patients with PS 0–1, stage IB to IIIA, within 10 weeks from surgery or within 26 weeks if they had had adjuvant chemotherapy. Patients were randomized 1:1 to either osimertinib or placebo for up to 3 years. The primary endpoint was Disease Free Survival (DFS). The unplanned efficacy interim assessment results were published after completing recruitment but only a median treatment of less than 2 years [22.5 (range, 0–38) months in the Osimertinib group and 18.7 (range, 0–36) months in the Placebo group]. All patients had been followed for at least 1 year. The median age was young, only 62 and 64 years old in the treatment and placebo arm respectively, and over 40% of patients had N0 disease in both arms. The number of patients recruited with tumours <40 mm in size and their outcome was not described. The DFS Hazard Ration (HR) for all patients with stage IB–IIIA was 0.2 (99.12% CI: 0.14–0.3). Forty-five patients out of the 682 had brain metastases at recurrence, mainly in the Placebo group. The CNS DFS (the reduction in the risk of recurrence in the brain) was 0.18 (95% CI: 0.10–0.33). Benefit was evident regardless of previous adjuvant chemotherapy, but results were immature to identify the added value of chemotherapy in osimertinib-treated patients. The lack of data maturity was evident in the heavily censored Kapplan-Meyer curves, as expected in an unplanned interim analysis. Interstitial lung disease was reported in 10 patients in the Osimertinib group (3%), and none in the Placebo group. Grade 3 or higher adverse events were reported in 20% of patients in the Osimertinib group and 13% in the Placebo group. Eleven percent of patients discontinued treatment due to adverse events in the Osimertinib group—a proportion that is considered acceptable when is less than 20%—and 3% in the Placebo group. Because of the impressive DFS HR and good treatment toleration, patients are currently offered adjuvant osimertinib for 3 years, as advised in updated guidelines (24). The impact of adjuvant osimertinib on OS (a secondary objective in the study), remains to be reported. Both patients and investigators remained unaware of their allocation and follow up for this study continues.
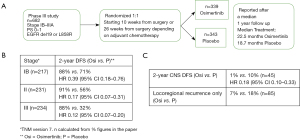
To anticipate what may happen when patients complete the 3 years of osimertinib, it is important to look at earlier adjuvant studies using EGFR inhibitors in patients with common EGFR mutations (L858R and deletion 19), where more mature data is available. Li et al. (25) reported the results of a single-centre study with 60 patients with stage IIIA-N2 randomized to carboplatin and pemetrexed followed by 6 months of gefitinib vs. adjuvant carboplatin and pemetrexed alone. The 2-year DFS was 78.9% in the gefitinib arm and 54.2% in the control arm. Another single-centre Phase II study (26) randomized 41 patients with stage IB to IIIA [17 (43.6%) of them with stage IB], to four cycles of adjuvant chemotherapy vs. chemotherapy starting at the same time as icotinib, which continued for up to eight months. Consistently, the 2-year DFS was 90.5% in the TKI group and 66.7% in the control group. These results suggest that DFS benefit may prevail beyond the TKI treatment period, but it remains to be seen if this will translate into survival benefit.
The most mature data in patients with common EGFR mutations is the CTONG-1104 study (27). Two hundred and twenty-two patients with stages II to IIIA-N2 (all lymph node-positive) were randomized to receive adjuvant platinum chemotherapy vs. two years of gefitinib without chemotherapy. The primary endpoint was DFS. After a median follow up of 36.5 months the DFS HR was 0.60 (95% CI: 0.42–0.87), with a median DFS of 28.7 (95% CI: 24.9–32.5) months for the TKI group vs. 18 (95% CI: 13.6–22.3) months for the chemotherapy group. Treatment-related discontinuation occurred in 3% of patients in the TKI group and 6% in the chemotherapy group. One patient discontinued gefitinib due to grade 4 respiratory failure. A post hoc analysis (28) revealed that intracranial recurrence was more common in patients treated with gefitinib [it should be noted that gefitinib has poorer CNS penetration than osimertinib (29)]. The highest peak for extracranial recurrence in the Gefitinib group was between 24 and 30 months from surgery. When the 5-year updated results were published (27), the previously identified benefit had vanished. The 5-year DFS was 22.6% and 23.2%, and OS HR 0.92 (95% CI: 0.62–1.37). Amongst the patients that underwent further oncology treatment on recurrence, the median OS of patients in the Gefitinib group was 57.4 months, and 51.9 months for patients in the chemotherapy group.
Whereas the frequency of EGFR mutations appears to be similar through all stages (30,31), other genetic drivers with lower incidence such as ELM4-ALK translocations are less common in early stage lung cancer (32,33). Hence, identification of benefit in these less frequent populations can be more challenging. Nevertheless, the ALINA study (34) is currently recruiting patients with completely resected ALK+ tumours, stage IB–IIIA and PS 0–1, and randomizes them 1:1 to receive alectinib vs. cisplatin-based adjuvant chemotherapy, and the ALCHEMIST study (35) is a basket study that randomizes patients with stage IB (>4 cm)-IIIA and ALK translocation to Crizotinib or observation, patients with EGFR-positive tumours to erlotinib vs. observation, and those with wild type tumours to adjuvant Nivolumab vs. observation.
In patients with RET translocation, another infrequent event, the LIBRETTO-432 study randomizes patients with stage IB–IIIA to selpercatinib vs. placebo (36).
Finally, other novel therapies such as the KRASG12C inhibitor Sotorasib has been approved in the advanced stage setting as a second-line treatment after immunotherapy or chemo-immunotherapy (37). This KRASG12C mutation has a similar prevalence than EGFR (38). Further exploration of this therapeutic target is needed in the early stage setting, alone or in combination with other adjuvant treatments such as chemotherapy and/or immunotherapy.
ICI
One year of atezolizumab immunotherapy after adjuvant chemotherapy, in stage II–IIIA tumours with a PD-L1 of 50% or more, may reduce the risk of disease recurrence according to the recently reported pre-planned interim analysis of the Impower 010 study (39,40) (See Figure 2). This trial selected patients with complete resection (most had a lobectomy), exposed to at least one cycle of cisplatin-based chemotherapy, and PS 0–1, without prior exposure to checkpoint inhibitor therapy, autoimmune disease, HIV or active hepatitis. Cancers with EGFR mutations (9%) and ALK translocations (5%) were included in the study. Patients were consented immediately after surgery and over 4% had disease progression during adjuvant chemotherapy. One thousand and five patients were randomized after chemotherapy 1:1 to 16 3-weekly cycles of atezolizumab (1 year) vs. active monitoring. Randomization took place 3–8 weeks from the last chemotherapy dose. Eight hundred and eighty-two patients had stage II–IIIA disease, of which 476 had PD-L1 positive disease. The primary endpoint was hierarchical investigator assessed DFS. The first effectiveness analysis was performed in 476 patients with PDL1 ≥1% and stage II–IIIA. This was the population for which the interim analysis was pre-planned once 190 DFS events had occurred amongst them, and the subgroup with most interesting results to date. After a median follow up of 32.2 months, the 3-year DFS was 60% in the Atezolizumab group vs. 48% in the best supportive care (BSC).
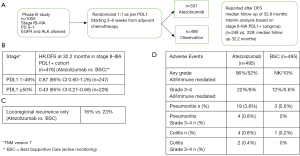
The DFS HR benefit seemed to be driven by the group with high-PDL1 expression. In an unplanned subgroup analysis, patients with PDL1 ≥50% (n=229), the DFS HR was 0.43 (95% CI: 0.27–0.68), and 0.87 (95% CI: 0.60–1.26) amongst patients with PDL1 1–49% (n=247). Further subgroup analysis showed no statistically significant benefit in patients with squamous cancers (n=294) nor in those without lymph node invasion (N0). Perhaps this data needs to mature further, but begs the question if this particular adjuvant immunotherapy alone is effective in squamous tumours. 11% of patients in the atezolizumab arm and 16% of those on the BSC group went on to have radiotherapy for local recurrence, whereas 5% vs. 7% respectively, had surgery. Although crossover was not allowed, more patients in the BSC arm (13%) went on to have palliative immunotherapy on progression than on the atezolizumab arm (4%), which may need to be considered when mature survival data is reported.
The treatment appeared tolerable. The safety population included 990 patients. Twenty-five percent of patients had 0–7 cycles of atezolizumab, 9% had 8–15 cycles and 65% had the planned 16 cycles. Ninety-five percent of patients on atezolizumab had adverse events of any grade and 18% of patients on atezolizumab had adverse events leading to treatment discontinuation.
As with TKI, the question remains if starting treatment on progression will have the same effect in patient survival and reduce the need to treat patients that will never have disease recurrence. Survival results are eagerly awaited.
EGFR mutations and ALK translocations were allowed in the study. Consistently with the advanced setting, subgroup analysis did not show a significant benefit. Adjuvant immunotherapy patients with non-smoking related driver mutations such as EGFR, ALK, ROS1, NTRK or RET may be futile, may increase TKI-toxicity (41), and in certain health systems may deprive them from accessing effective immunotherapy combination after TKI treatment (42).
Following the path set by the advanced stage, adjuvant trials using chemotherapy or targeted therapy combination with immunotherapy in patients with PDL1 0–49%, are expected.
Unfortunately, the study design of Impower 010 excluded patients with Cisplatin contra-indication or those that declined cytotoxic chemotherapy but may have considered adjuvant immunotherapy. This would have also helped to investigate the influence of chemotherapy in the context of adjuvant immunotherapy. Several phase III studies in this setting with Pembrolizumab (43) (Keaynote-091 PEARLS), Nivolumab (44) (ANVIL) and Durvalumab (45) (BR.31) are awaiting to be reported, all of them also after exposure to adjuvant chemotherapy. Further studies without adjuvant chemotherapy, at least in patients with high PDL1, are needed.
The neoadjuvant setting
The pre-operative setting has historically been attractive to obtain pre and post-treatment biological samples in early-phase hypothesis-generating studies (46). In chemotherapy studies, overall survival has shown good correlation with pathological complete response (pCR) (47,48) and major pathological response (47) (MPR; defined as the proportion of patients with 10% or less viable cancer tissue in the surgical sample), so clinical benefit could be reported much earlier than in the adjuvant setting. There has been only one large phase III study comparing neoadjuvant chemotherapy vs. adjuvant chemotherapy (49). More patients in the neoadjuvant group received chemotherapy, and their response correlated with time to recurrence. Recruitment in this setting, however, has been challenging. Pre-surgical biopsies have been historically done at the discretion of the institution, as retrospective series reported no influence in patient outcome (50). A delayed time to surgery due to adverse events from oncology treatment, limitations in diagnostic service provision, and the possibility of progression during neoadjuvant treatment may have challenged recruitment in this setting.
As novel treatments with low short-term toxicity and higher disease control rates are being tested, the possibility of increasing the chances of cure have made the neoadjuvant setting more attractive (51). This has benefited from an increased trend to perform pre-surgical biopsies, although assuming that short-term outcomes are still valid surrogate markers of survival when using novel targeted agents.
TKI
There is no current neoadjuvant standard of care in the management of mutation-driven lung cancers, but several studies are now recruiting. The NeoADAURA study (52) selects patients eligible to surgical resection with a common EGFR mutation (L858R or deletion 19), stage II–IIIB N2 [according to the 8th TNM edition (53)] and PS 0–1. Patients with T4 tumours infiltrating the aorta, oesophagus and/or the heart, or those with bulky N2 disease, are excluded. Patients are randomized 1:1:1 to 9 weeks of osimertinib, vs. 3 cycles of chemotherapy with osimertinib, vs. 3 cycles of chemotherapy with placebo. Surgery shall occur within 12 weeks from the start of neoadjuvant treatment. The primary objective is MPR. This study will collect samples for MRD [molecular residual disease (54) or minimal residual disease (55)] and ctDNA analysis (56) at several timepoints. This may allow short-outcome comparison between treatment arms—could MRD be a new short-term outcome in surgical patients?—as well as correlation with long term clinical outcomes. Patient access to 3 years of adjuvant osimertinib is assumed to be standard of care regardless of the treatment arm. Long-term outcomes and recurrence patterns are thus likely to be influenced by the effect of the adjuvant treatment, but the set up will allow correlation between post-surgical MRD results and likelihood of recurrence after adjuvant therapy.
Crizotinib is a multitarget TKI that can inhibit ALK and ROS1 translocations as well as MET exon 14 skipping mutation. Feasibility has been tested in a small study (n=11) in the neoadjuvant setting in patients with ALK translocations (57). Currently, there is a single-arm phase II study in the neoadjuvant setting recruiting lung cancer patients with ALK/ROS1 translocations and MET exon 14 skipping mutation (58), and stage IA–IIIA, where patients are exposed to 6 weeks of crizotinib before surgery. The primary endpoint is objective response rate (ORR). The study phase, lack of randomization, and multiple mutation options is an indication of the challenges in early-stage research for these low-frequency translocations.
ICI
A theoretical advantage of using immunotherapy in the neoadjuvant setting over the adjuvant setting is that cell death releases cancer antigens into the bloodstream that stimulate the immune response. Immune activation may be more relevant in the longer term if the treatment is given when the tumour is still in situ (59). Since the benefit of adjuvant immunotherapy is most prominent in patients with high PDL1 levels, treatment in the neoadjuvant setting may be especially relevant in patients with PDL1 0–49%. There are multiple neoadjuvant early-phase studies with single-agent immunotherapy and chemotherapy combination (60). Pathological Complete Response (pCR) rates of chemo-immunotherapy appear much higher than with immunotherapy alone. For example, pCR was achieved in 9% of patients with stages I–IIIA N2 treated with neoadjuvant Nivolumab (n=2/23), and 29% of those treated with Nivolumab and Ipilimumab (n=6/21) within the NEOSTAR trial (61). Nonetheless, combining immunotherapy with chemotherapy before surgical intervention may be more effective. In the NADIM study (62), 46% of patients with stage IIIA (n=21/46) had pCR. This dramatic increase in pCR explains why most of the ongoing phase III studies are testing chemo-immunotherapy combinations.
The Keynote 671 study (63) includes patients with stage II–IIIB and randomizes them to neoadjuvant chemotherapy with Pembrolizumab or Placebo. It aims to recruit 786 patients. The primary endpoints are event-free survival (EFS) and OS. The Checkmate 816 study (64) selects patients with stage IB (≥4 cm)–IIIA and randomizes them to chemotherapy vs. chemotherapy and nivolumab, vs. nivolumab with ipilimumab. It aims to recruit 350 patients. The primary endpoints are EFS and pCR. Surgical outcomes of chemotherapy vs. chemotherapy and nivolumab (n=179 in each arm) were reported in ASCO 2021 (65). More patients in the chemo-immunotherapy arm completed the planned neoadjuvant treatment (94% vs. 85%). Surgery cancellations were lower (16% vs. 21%), as well as the median duration of surgery (184 vs. 217 min). More patients had minimally invasive surgery (30% vs. 22%), and less patients changed from minimally invasive to open surgery (11% vs. 16%). Consistently, less patients in the Nivolumab-chemotherapy arm had a pneumonectomy (17% vs. 25%). Amongst the biggest differences in 90-day surgery-related complications, pain (7% vs. 16%), cough (2% vs. 4%), nausea (1% vs. 3%), dyspnoea (1% vs. 4%) and pulmonary fistula (1% vs. 3%) were more common in patients that received chemotherapy alone, whereas wound complications (8% vs. 6%) and pyrexia (5% vs. 2%) where more common in patients treated with chemo-immunotherapy. pCR rates were 40% in stage IB and 23–24% in stages II and IIIA, compared with 0% and 1–9% with chemotherapy alone, respectively. These numbers put the results of the smaller NADIM study in a different perspective: not half, but almost a quarter of patients had no residual disease on pathological assessment in the Checkmate 816 study after chemo-immunotherapy.
Interestingly, the rest of ongoing neoadjuvant phase III studies focus on the higher-stage operable disease and do not include stage I disease. Impower 030 (66) selects patients with stage II–IIIB and randomizes them to chemotherapy and atezolizumab vs. chemotherapy and Placebo. Its Primary Endpoint is MPR and EFS. The Checkmate 77T with Nivolumab (67) also selects patients with stage IIA–IIIB (T3 N2 only), and the AEGEAN study (68) uses Durvalumab and selects patients with stage IIA–IIIB.
All these phase III neoadjuvant studies, except for the Checkmate 816 study, have an adjuvant immunotherapy component of between 6 months to a year.
Another possible strategy in the neoadjuvant setting that is not possible after surgery, is the combination of immunotherapy with radiotherapy (69). Radiation may provide an “abscopal effect” of immunotherapy (70). This posits that local antigen release promotes immune system activation, similar to the effect of chemotherapy but with only local treatment. The main concern of this strategy in lung cancer patients is pneumonitis (71), but a small real-life series (72) showed low rates of pneumonitis (7%), mainly of grade 1–2, with higher chances of oesophagitis in doses of >3,000 cGy. Anyhow, scarring, oedema and delayed radiotherapy toxicity may increase the challenge of surgical intervention, and previous studies with neoadjuvant radiotherapy have shown reduction in local recurrence, but not in improved resectability or long-term outcomes (73,74). It is apparent that so far this approach has not taken off, as ongoing phase III studies in the neoadjuvant setting only appear to combine immunotherapy with chemotherapy, but with the recent improvement of radiotherapy delivery options and the increased availability of scans that can identify metabolically-active areas (PETCT), strategies to potentiate the abscopal effect and minimize toxicity such as partial irradiation for large tumours in the neoadjuvant setting could potentially be integrated into immunotherapy or chemo-immunotherapy regimens in the future.
Discussion
There are recent excellent reviews of targeted treatments in this setting (60,75,76), but this is a rapidly evolving field. We expanded here on studies that have been published or reported since, and in this Discussion section we aim to underline the evidence related to the possible impact on service provision, patient selection and follow up considerations.
Adjuvant setting
There are many uncertainties around the use of adjuvant osimertinib in patients with common EGFR mutations. First, there is a lack of objective patient selection markers. Efforts are necessary to identify possible predictive markers of recurrence such as co-mutations [i.e., the presence of p53 mutations in the advanced setting is a poor prognostic biomarker (77)], or direct diagnostic markers such as residual molecular disease and early microscopic recurrence (54) in these patients, so that cured patients can avoid treatment exposure and health systems can optimize expenses. Whether patients with proven molecular residual disease or early microscopic recurrence will be curable or not, remains to be seen.
Although the benefit in delaying recurrence is obvious, as data matures, more evidence may be able to identify progression patterns to inform patient follow up. In the meantime, it is not unreasonable to continue as per current guidelines but including brain imaging, while being mindful that, as it happened in the adjuvant gefitinib study (27,28), a cohort of patients may progress shortly after discontinuation of the 3-year treatment. Although radical treatment of local recurrence in this patient group may be possible, ongoing TKI continuation may be necessary.
Our hospital guidelines indicate 6-monthly CT chest and liver with brain MRI while the patient is on osimertinib, followed by 3-monthly imaging for 2 years, but this is a local choice influenced by our current service provision limitations. There is a need to provide the evidence to develop adjuvant follow up guidelines in this patient population beyond 5 years, who in the absence of smoking-related co-morbidities and a larger young patient subgroup at diagnosis, may follow a very different recurrence pattern than patients with wild type lung cancer.
As it was mentioned earlier, adjuvant early phase studies (25,26) have shown some prolonged benefit of its cytotoxic activity beyond the treatment period, but the fact that survival curves joined back in the large phase III study (28) suggests that the benefit may be short-lived. OS data of the ADAURA study is eagerly awaited. Adjuvant osimertinib, nevertheless, may be particularly attractive in the elderly population, who may benefit the most from avoiding symptomatic cancer-recurrence, especially within the CNS. In view of the low average age of patients within the ADAURA study, further tolerability and quality of life research in the elderly subgroup may contribute to inform treatment choice.
To our knowledge, there is no published evidence on the effect of adjuvant chemotherapy in patients with an EGFR mutation. As the control arm of the ADAURA data matures, this may be a helpful question that this study may also answer—patients could avoid chemotherapy altogether. Alternatively, if chemotherapy is found to be beneficial, it could be helpful to test in future studies the effect of adjuvant osimertinib alongside chemotherapy, which may at least reduce follow up time and costs.
From now on, all resected tumours with at least pT2a pN0 stage will need EGFR testing, ideally as part of NGS mutation and translocation panels. In this regard, strategies to support molecular testing to all patients, such as the nation-wide genetic service implementation in England (78), are key. This has centralized molecular testing in seven Genetic Hubs across England to maximise the possibilities of molecular testing. The caveat is that as technology evolves, local testing in the not-so-distant future may be feasible, reducing resource utilisation, optimizing sample travel, and providing faster results. Anyhow, a centralized set up offers the chance of building a consistent, large genetic panel data, and the possibility to agree a consensus for prospective data collection in patients with genetic drivers that may facilitate further understanding of their clinical evolution from the early stage setting.
It shall be mentioned that due to the radically different mechanisms of action between TKI and chemotherapy, the studies that obviate chemotherapy in the experimental group such as the reported adjuvant Gefitinib study (27,28) or the currently recruiting ALINA study (34), are missing the chance to explore the possibility of cytotoxic synergy. Their long-term outcomes cannot be directly compared with randomized studies that allow adjuvant chemotherapy in both arms.
Adjuvant immunotherapy is now standard of care. The results of the Impower 010 study (39,40) suggest that the benefit is driven by patients with high PDL1 expression, hence it will be key to have PDL1 results in all surgical specimens amenable to adjuvant treatment. Further translational studies may be able to identify the patient subpopulation within the PDL1 1–49% subgroup that benefited from adjuvant immunotherapy. In the meantime, replicating the extensive disease setting, it may be helpful to explore the benefit of adjuvant or neoadjuvant concurrent chemo-immunotherapy in patients with PDL1 <50%; or the effect of giving adjuvant immunotherapy without previous adjuvant chemotherapy in patients with PDL1 ≥50%. Other lessons should also be learnt from the advanced setting, where several immunotherapy drugs appeared in the market within a short time-period for the same lung cancer patient population. Due to the lack of direct immunotherapy drug comparisons, drug choice in second-line single-agent immunotherapy was based on drug schedules, price, and indirect subgroup analysis comparison, rendering choice more susceptible to the efficacy of marketing campaigns and speed of drug approval instead of clinical evidence of efficacy. Again, direct immunotherapy drug comparison studies in the early-stage setting are unlikely to happen soon. Subgroup analysis, even if it was not pre-planned, should include outcome results in patients with PDL1 ≥50%, 1–49% and 0%, smokers and never-smokers, as well as in squamous vs. non-squamous subtypes, and a more extensive description of the timing of immunotherapy adverse events. Perhaps standardized trial reporting guidelines for immunotherapy studies may aid clinicians guide drug choice and follow up arrangements around high-incidence timepoints.
There are many unanswered questions in this setting that will be amenable to further research. Early and late recurrence patterns of these patients may differ substantially from those previously treated with chemotherapy. It can be assumed that local recurrences will be treated with radical radiotherapy while continuing adjuvant immunotherapy, but the length of treatment after local recurrence may be then questioned—should patients with local recurrence complete a 2-year immunotherapy course? If so, prospective evidence may be necessary for funding bodies take this treatment plan switch into account. Finally, patients with distant recurrence within a year of platinum chemotherapy may only be amenable to second-line chemotherapy or BSC. Alternatives in patients with primary resistance to immunotherapy are needed, and consensus definitions of primary and acquired immunotherapy resistance may contribute to focus translational and clinical research.
Overall, in view of the 4.4% progression rate on chemotherapy, repeat imaging before engaging with adjuvant immunotherapy may be advisable. To identify early the 40% of patients that progressed during the first 3 years and monitor pulmonary toxicity, we propose 3-monthly imaging during this time. As toxicity and progression data evolves, and high-risk follow up periods are identified, evidence-based guidelines will be possible. The projected service pressures on radiology departments will need to be considered and funded accordingly.
Again, it is important to underline the need for patient selection markers in the adjuvant setting such as residual molecular disease or ctDNA. This will be even more relevant if neoadjuvant treatment becomes standard of care, as the proportion of cured patients exposed to unnecessary adjuvant treatments may increase if neoadjuvant treatments are successful.
All this suggests that heavy investment in lung pathology and molecular testing will continue to be key as targeted treatments continue to invade the early-stage disease.
Neoadjuvant setting
The biggest challenge of the neoadjuvant setting is to obtain enough tissue for molecular diagnosis in diagnostic samples. In some cancer types such as the ALK+, the low incidence in the early stage is an additional challenge. Not all patients will be amenable to pre-surgical biopsies but for those who are, if the neoadjuvant setting consolidates within standard of care, it will be important to increase and homogenise interventional radiology and diagnostic respiratory service provision across the health networks. During neoadjuvant treatment, it is necessary to establish good communication channels between the oncology and surgical teams to ensure optimal treatment support and swift access to surgical intervention. Multidisciplinary models of care will be key (79).
Finally, it shall be underlined again that most neoadjuvant chemo-immunotherapy studies have an adjuvant immunotherapy phase. The argument to continue treatment after surgery may be to consolidate the treatment of post-surgical micrometastatic disease, but somehow this defies the purpose of the neoadjuvant setting, where surgical cures may be increased and hence a larger amount of patients may be able to avoid post-surgical treatment. Biomarkers of residual disease or microscopic recurrence may contribute to identify this patient population. In the meantime, indirect comparison of the Checkmate 816 study (65) (without adjuvant immunotherapy) and the Checkmate 77T (67) (with adjuvant immunotherapy), may provide an indication of the potential effect of post-surgical immunotherapy.
Patient selection
While technology evolves and molecular markers of residual disease develop, we shall focus on the available patient selection criteria, particularly PS and disease stage.
Most of the neoadjuvant and adjuvant studies select patients with PS 0–1, but there is a proportion of patients with PS 2 that are amenable to surgical intervention. Smoking-related co-morbidities with PS limitations are not uncommon in the lung cancer population. It is important to cater for all and ensure that lower-toxicity therapies are accessible to them, as this subgroup may notice a bigger benefit in delaying recurrence than patients that will be amenable to all treatment options on recurrence. Patients with PS 2 after surgical intervention, for whom adjuvant chemotherapy was detrimental, represent a population of unmet treatment need that may benefit the most from exploring adjuvant single agent immunotherapy or TKI.
Adjuvant studies also excluded patients that had a segmentegtomy, wedge resection or residual (≥R1) disease. Prospective investigation of adjuvant targeted treatment (with or without radiotherapy) in these patients may also identify a possible benefit.
In view of the excellent tumour volume reduction with neoadjuvant chemo-immunotherapy (65), it may be worth a further exploration of borderline stage III patients, and why not, even in local inoperable disease. There is a need to break down and re-define stage III disease to test these new therapies, discerning subgroups that may benefit from downstaging and accessing surgical options.
Conclusions
Novel targeted therapies represent a paradigm shift in the management of early-stage lung cancer. Prospective research is needed to optimize patient selection. Circulating tumour DNA analysis may play an important part in identifying residual molecular disease and early recurrence, which may help select patients that may benefit from adjuvant treatment. Early-stage studies need to tailor their follow up considering the need to report on recurrence and adverse event patterns that may inform follow up guidelines. Finally, centres may need to adapt their service to optimize pre-surgical diagnosis and post-surgical molecular pathology profiling.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Michael Shackcloth and Amer Harky) for the series “Lung Cancer Surgery” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at https://vats.amegroups.com/article/view/10.21037/vats-21-39/rc
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-21-39/coif). The series “Lung Cancer Surgery” was commissioned by the editorial office without any funding or sponsorship. The author has received lecturing fees from Astrazeneca, BMS, Pfizer, Amgen, MSD, Eli Lilly and Roche, and is member of the Steering Committee for the NeoADAURA study.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Brózik A, Hegedüs C, Erdei Z, et al. Tyrosine kinase inhibitors as modulators of ATP binding cassette multidrug transporters: substrates, chemosensitizers or inducers of acquired multidrug resistance? Expert Opin Drug Metab Toxicol 2011;7:623-42. [Crossref] [PubMed]
- Cohen P, Cross D, Jänne PA. Kinase drug discovery 20 years after imatinib: progress and future directions. Nat Rev Drug Discov 2021;20:551-69. [Crossref] [PubMed]
- Kubli SP, Berger T, Araujo DV, et al. Beyond immune checkpoint blockade: emerging immunological strategies. Nat Rev Drug Discov 2021;20:899-919. [Crossref] [PubMed]
- Mo J, Hu X, Gu L, et al. Smokers or non-smokers: who benefits more from immune checkpoint inhibitors in treatment of malignancies? An up-to-date meta-analysis. World J Surg Oncol 2020;18:15. [Crossref] [PubMed]
- Zahreddine H, Borden KL. Mechanisms and insights into drug resistance in cancer. Front Pharmacol 2013;4:28. [Crossref] [PubMed]
- White MN, Piotrowska Z, Stirling K, et al. Combining Osimertinib With Chemotherapy in EGFR-Mutant NSCLC at Progression. Clin Lung Cancer 2021;22:201-9. [Crossref] [PubMed]
- Asahina H, Tanaka K, Morita S, et al. A Phase II Study of Osimertinib Combined With Platinum Plus Pemetrexed in Patients With EGFR-Mutated Advanced Non-Small-cell Lung Cancer: The OPAL Study (NEJ032C/LOGIK1801). Clin Lung Cancer 2021;22:147-51. [Crossref] [PubMed]
- ClinicalTrials.gov. A Study of Osimertinib With or Without Chemotherapy as 1st Line Treatment in Patients With Mutated Epidermal Growth Factor Receptor Non-Small Cell Lung Cancer (FLAURA2). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04035486
- Chapman AM, Sun KY, Ruestow P, et al. Lung cancer mutation profile of EGFR, ALK, and KRAS: Meta-analysis and comparison of never and ever smokers. Lung Cancer 2016;102:122-34. [Crossref] [PubMed]
- Shin DY, Na II, Kim CH, et al. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol 2014;9:195-9. [Crossref] [PubMed]
- Calles A, Riess JW, Brahmer JR. Checkpoint Blockade in Lung Cancer With Driver Mutation: Choose the Road Wisely. Am Soc Clin Oncol Educ Book 2020;40:372-84. [Crossref] [PubMed]
- Isaka T, Nakayama H, Ito H, et al. Impact of the epidermal growth factor receptor mutation status on the prognosis of recurrent adenocarcinoma of the lung after curative surgery. BMC Cancer 2018;18:959. [Crossref] [PubMed]
- Wu SG, Liao WY, Su KY, et al. Prognostic Characteristics and Immunotherapy Response of Patients With Nonsquamous NSCLC With Kras Mutation in East Asian Populations: A Single-Center Cohort Study in Taiwan. JTO Clin Res Rep 2021;2:100140. [Crossref] [PubMed]
- Fujino T, Suda K, Mitsudomi T. Lung Cancer with MET exon 14 Skipping Mutation: Genetic Feature, Current Treatments, and Future Challenges. Lung Cancer (Auckl) 2021;12:35-50. [Crossref] [PubMed]
- Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer 2019;7:278. [Crossref] [PubMed]
- Haragan A, Field JK, Davies MPA, et al. Heterogeneity of PD-L1 expression in non-small cell lung cancer: Implications for specimen sampling in predicting treatment response. Lung Cancer 2019;134:79-84. [Crossref] [PubMed]
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. [Crossref] [PubMed]
- Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 1995;311:899-909. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Salazar MC, Rosen JE, Wang Z, et al. Association of Delayed Adjuvant Chemotherapy With Survival After Lung Cancer Surgery. JAMA Oncol 2017;3:610-9. [Crossref] [PubMed]
- Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:1711-23. [Crossref] [PubMed]
- Remon J, Soria JC, Peters S, et al. Early and locally advanced non-small-cell lung cancer: an update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol 2021;32:1637-42. [Crossref] [PubMed]
- Li N, Ou W, Ye X, et al. Pemetrexed-carboplatin adjuvant chemotherapy with or without gefitinib in resected stage IIIA-N2 non-small cell lung cancer harbouring EGFR mutations: a randomized, phase II study. Ann Surg Oncol 2014;21:2091-6. [Crossref] [PubMed]
- Feng S, Wang Y, Cai K, et al. Randomized Adjuvant Chemotherapy of EGFR-Mutated Non-Small Cell Lung Cancer Patients with or without Icotinib Consolidation Therapy. PLoS One 2015;10:e0140794. [Crossref] [PubMed]
- Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol 2018;19:139-48. [Crossref] [PubMed]
- Xu ST, Xi JJ, Zhong WZ, et al. The Unique Spatial-Temporal Treatment Failure Patterns of Adjuvant Gefitinib Therapy: A Post Hoc Analysis of the ADJUVANT Trial (CTONG 1104). J Thorac Oncol 2019;14:503-12. [Crossref] [PubMed]
- Ballard P, Yates JW, Yang Z, et al. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin Cancer Res 2016;22:5130-40. [Crossref] [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Tabbò F, Nottegar A, Guerrera F, et al. Cell of origin markers identify different prognostic subgroups of lung adenocarcinoma. Hum Pathol 2018;75:167-78. [Crossref] [PubMed]
- Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res 2009;15:5216-23. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [Crossref] [PubMed]
- Solomon BJ, Ahn JS, Barlesi F, et al. ALINA: A phase III study of alectinib versus chemotherapy as adjuvant therapy in patients with stage IB–IIIA anaplastic lymphoma kinase-positive (ALK+) non-small cell lung cancer (NSCLC). J Clin Oncol 2019;37:TPS8569. [Crossref]
- Sands J, Mandrekar SJ, Oxnard GR, et al. ALCHEMIST: Adjuvant targeted therapy or immunotherapy for high-risk resected NSCLC. J Clin Oncol 2020;38:TPS9077. [Crossref]
- Goldman J, Besse B, Wu Y, et al. P01.01 LIBRETTO-432: A Placebo-Controlled Phase 3 Study of Adjuvant Selpercatinib in Stage IB-IIIA RET Fusion-Positive NSCLC. J Thorac Oncol 2021;16:S975-6. [Crossref]
- Skoulidis F, Li BT, Dy GK, et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N Engl J Med 2021;384:2371-81. [Crossref] [PubMed]
- Zhang SS, Nagasaka M. Spotlight on Sotorasib (AMG 510) for KRAS G12C Positive Non-Small Cell Lung Cancer. Lung Cancer (Auckl) 2021;12:115-22. [Crossref] [PubMed]
- Zhou C, Altorki N, Valliéres E, et al. 429TiP IMpower010: A Phase III trial investigating atezolizumab (atezo) vs best supportive care (BSC) after adjuvant chemotherapy (chemo) in patients (pts) with completely resected NSCLC. Ann Oncol 2016;27:IX135. [Crossref]
- Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021;398:1344-57. [Crossref] [PubMed]
- Schoenfeld AJ, Arbour KC, Rizvi H, et al. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann Oncol 2019;30:839-44. [Crossref] [PubMed]
- Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019;7:387-401. [Crossref] [PubMed]
- ClinicalTrials.gov. Study of Pembrolizumab (MK-3475) vs Placebo for Participants With Non-small Cell Lung Cancer After Resection With or Without Standard Adjuvant Therapy (MK-3475-091/KEYNOTE-091). Available online: https://clinicaltrials.gov/ct2/show/NCT02504372
- ClinicalTrials.gov. Nivolumab After Surgery and Chemotherapy in Treating Patients With Stage IB-IIIA Non-small Cell Lung Cancer (An ALCHEMIST Treatment Trial). Available online: https://clinicaltrials.gov/ct2/show/NCT02595944
- ClinicalTrials.gov. Double Blind Placebo Controlled Controlled Study of Adjuvant MEDI4736 In Completely Resected NSCLC. Available online: https://clinicaltrials.gov/ct2/show/NCT02273375
- Hayes DF, Schott AF. Neoadjuvant Chemotherapy: What Are the Benefits for the Patient and for the Investigator? J Natl Cancer Inst Monogr 2015;2015:36-9. [Crossref] [PubMed]
- Hellmann MD, Chaft JE, William WN Jr, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014;15:e42-50. [Crossref] [PubMed]
- Huynh C, Sorin M, Rayes R, et al. Pathological complete response as a surrogate endpoint after neoadjuvant therapy for lung cancer. Lancet Oncol 2021;22:1056-8. [Crossref] [PubMed]
- Felip E, Rosell R, Maestre JA, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol 2010;28:3138-45. [Crossref] [PubMed]
- Taniguchi Y, Fukumoto K, Matsui H, et al. Preoperative biopsy does not affect postoperative outcomes of resectable non-small cell lung cancer. Gen Thorac Cardiovasc Surg 2019;67:615-23. [Crossref] [PubMed]
- Blumenthal GM, Bunn PA Jr, Chaft JE, et al. Current Status and Future Perspectives on Neoadjuvant Therapy in Lung Cancer. J Thorac Oncol 2018;13:1818-31. [Crossref] [PubMed]
- Tsuboi M, Weder W, Escriu C, et al. Neoadjuvant osimertinib with/without chemotherapy versus chemotherapy alone for EGFR-mutated resectable non-small-cell lung cancer: NeoADAURA. Future Oncol 2021;17:4045-55. [Crossref] [PubMed]
- Rami-Porta R, Asamura H, Travis WD, et al. Lung cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:138-55.
- Coakley M, Garcia-Murillas I, Turner NC. Molecular Residual Disease and Adjuvant Trial Design in Solid Tumors. Clin Cancer Res 2019;25:6026-34. [Crossref] [PubMed]
- Moding EJ, Nabet BY, Alizadeh AA, et al. Detecting Liquid Remnants of Solid Tumors: Circulating Tumor DNA Minimal Residual Disease. Cancer Discov 2021;11:2968-86. [Crossref] [PubMed]
- Escriu C, Field JK. Circulating tumour DNA and resistance mechanisms during EGFR inhibitor therapy in lung cancer. J Thorac Dis 2016;8:2357-9. [Crossref] [PubMed]
- Zhang C, Li SL, Nie Q, et al. Neoadjuvant Crizotinib in Resectable Locally Advanced Non-Small Cell Lung Cancer with ALK Rearrangement. J Thorac Oncol 2019;14:726-31. [Crossref] [PubMed]
- ClinicalTrials.gov. Evaluating Crizotinib in the Neoadjuvant Setting in Patients With Non-small Cell Lung Cancer. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03088930
- Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol 2020;20:651-68. [Crossref] [PubMed]
- Ortega-Franco A, Calvo V, Franco F, et al. Integrating immune checkpoint inhibitors and targeted therapies in the treatment of early stage non-small cell lung cancer: a narrative review. Transl Lung Cancer Res 2020;9:2656-73. [Crossref] [PubMed]
- Cascone T, William WN Jr, Weissferdt A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med 2021;27:504-14. [Crossref] [PubMed]
- Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:1413-22. [Crossref] [PubMed]
- ClinicalTrials.gov. Efficacy and Safety of Pembrolizumab (MK-3475) With Platinum Doublet Chemotherapy as Neoadjuvant/Adjuvant Therapy for Participants With Resectable Stage II, IIIA, and Resectable IIIB (T3-4N2) Non-small Cell Lung Cancer (MK-3475-671/KEYNOTE-671). Available online: https://clinicaltrials.gov/ct2/show/NCT03425643
- ClinicalTrials.gov. A Neoadjuvant Study of Nivolumab Plus Ipilimumab or Nivolumab Plus Chemotherapy Versus Chemotherapy Alone in Early Stage Non-Small Cell Lung Cancer (NSCLC). Available online: https://clinicaltrials.gov/ct2/show/NCT02998528
- Spicer J, Wang C, Tanaka F, et al. Surgical outcomes from the phase 3 CheckMate 816 trial: Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo alone as neoadjuvant treatment for patients with resectable non-small cell lung cancer (NSCLC). J Clin Oncol 2021;39:8503. [Crossref]
- Peters S, Kim AW, Solomon B, et al. 82TIP IMpower030: Phase III study evaluating neoadjuvant treatment of resectable stage II-IIIB non-small cell lung cancer (NSCLC) with atezolizumab (atezo) + chemotherapy. Ann Oncol 2019;30:ii30. [Crossref]
- Cascone T, Provencio M, Sepesi B, et al. Checkmate 77T: A phase III trial of neoadjuvant nivolumab (NIVO) plus chemotherapy (chemo) followed by adjuvant nivo in resectable early-stage NSCLC. J Clin Oncol 2020;38:TPS9076. [Crossref]
- Heymach J, Taube J, Mitsudomi T, et al. P1.18-02 The AEGEAN Phase 3 Trial of Neoadjuvant/Adjuvant Durvalumab in Patients with Resectable Stage II/III NSCLC. J Thorac Oncol. 2019;14:S625-6. [Crossref]
- Taunk NK, Rimner A, Culligan M, et al. Immunotherapy and radiation therapy for operable early stage and locally advanced non-small cell lung cancer. Transl Lung Cancer Res 2017;6:178-85. [Crossref] [PubMed]
- Grass GD, Krishna N, Kim S. The immune mechanisms of abscopal effect in radiation therapy. Curr Probl Cancer 2016;40:10-24. [Crossref] [PubMed]
- Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys 2005;63:5-24. [Crossref] [PubMed]
- von Reibnitz D, Wu AJ, Barker CA, et al. Safety of combining immune checkpoint inhibition and thoracic radiation therapy. Int J Radiat Oncol Biol Phys. 2016;96:S156. [Crossref]
- Bromley LL, Szur L. Combined radiotherapy and resection for carcinoma of the bronchus; experiences with 66 patients. Lancet 1955;269:937-41. [Crossref] [PubMed]
- Shields TW, Higgins GA Jr, Lawton R, et al. Preoperative x-ray therapy as an adjuvant in the treatment of bronchogenic carcinoma. J Thorac Cardiovasc Surg 1970;59:49-61. [Crossref] [PubMed]
- Friedlaender A, Addeo A, Russo A, et al. Targeted Therapies in Early Stage NSCLC: Hype or Hope? Int J Mol Sci 2020;21:6329. [Crossref] [PubMed]
- Chaft JE, Rimner A, Weder W, et al. Evolution of systemic therapy for stages I-III non-metastatic non-small-cell lung cancer. Nat Rev Clin Oncol 2021;18:547-57. [Crossref] [PubMed]
- Hou H, Qin K, Liang Y, et al. Concurrent TP53 mutations predict poor outcomes of EGFR-TKI treatments in Chinese patients with advanced NSCLC. Cancer Manag Res 2019;11:5665-75. [Crossref] [PubMed]
- NHS England. NHS Genomic Medicine Service. Available online: https://www.england.nhs.uk/genomics/nhs-genomic-med-service/
- Hardavella G, Frille A, Theochari C, et al. Multidisciplinary care models for patients with lung cancer. Breathe (Sheff) 2020;16:200076. [Crossref] [PubMed]
Cite this article as: Escriu C. Role and evidence for targeted therapies in surgically resectable non-small cell lung cancer: a narrative review. Video-assist Thorac Surg 2022;7:11.


