
Re-operative surgery after paraesophageal hernia repair: narrative review
Introduction
Paraesophageal hernia repairs are increasingly being approached through minimally invasive techniques. Rates of recurrence vary with 5–59% reported in the current literature (1) however not all recurrences require surgery. Revisional procedures for recurrent paraesophageal hernias are estimated to occur in 15% of patients (2,3). These surgeries are fraught with increased morbidity (15–40%) and mortality (0–2%) secondary to the heightened complexity of reoperations for even the most experienced foregut surgeons. Distorted anatomy, adhesions and possible presence of mesh increases risk of esophageal perforation, gastric perforation, vagal nerve injury and splenic trauma. Successful symptom control from re-operative surgery is found in less than 80% of patients compared to over 90% in primary surgery (4).
We present the following article in accordance with the Narrative Review reporting checklist (available at https://vats.amegroups.com/article/view/10.21037/vats-21-31/rc).
Types of recurrence
Early recurrences (<6 months) are usually technical in nature or from inappropriate patient selection. Late recurrences (>6 months) may be related to the weakness of the diaphragm and patient factors such as advanced age, increased body mass index (BMI) and activity level. All recurrent paraesophageal hernias do not need operative intervention. Radiological recurrences defined as recurrent hernias on imaging such as computed tomography (CT) scan or esophagogram with no significant symptoms can be observed. Interpretation of esophageal imaging after foregut surgery needs expertise as previous wedge gastroplasty can be misread as a recurrence.
Small recurrences with minimal symptoms can be treated medically with dietary changes or anti-reflux medications. In our practice we follow patients with yearly esophagograms which permits close surveillance (Figure 1). Significant or progressive anatomic recurrence in association with symptoms need operative repair.
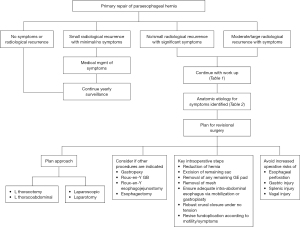
One can describe the nature of recurrence based on the location of the gastroesophageal junction (GEJ), the fundoplication (wrap) and the body of the stomach in relation to the esophageal hiatus. For the purpose of generalization, there are four ways in which the post-operative anatomy can be distorted. These are (I) the wrap is in the normal subdiaphragmatic location with the herniation of the GEJ and cardia through the wrap into the chest; (I) the GEJ is subdiaphragmatic but the wraps slips down onto the cardia; (III) the GEJ and the wrap slip through the hiatus and become supra-diaphragmatic; and (IV) the GEJ and the wrap are subdiaphragmatic but the body of the stomach or other abdominal viscera herniate into the chest adjacent to the wrap. Understanding the type of recurrence is important in attempting to repair it.
Indications for revisional surgery
Recurrence of the hiatal hernia is likely the most common indication for revision after a prior paraesophageal hernia repair. However, other indications for re-operative surgery that do not involve a hernia is an improper wrap construction that is either too tight, torsed, or a wrap that involves the gastric body rather than the fundus. These minute anatomic derangements can produce significant dysphagia and abdominal pain.
Recurrences generally involve mediastinal migration of the wrap (5). Possible etiologies for wrap hernia include incorrect wrap construction, incomplete removal of the previous hernia sac and failure to perform a complete mediastinal dissection or a lengthening procedure (such as a wedge gastroplasty or Collis gastroplasty) to address a “short esophagus”.
Recurrent hernia due to improper hiatal closure could be attributable to a loose wide hiatus, degloving of the peritoneum on the crura that prevents effective suture retention, and increased crural tension with failure to perform relaxing diaphragm incisions.
Missed diagnoses of dysmotility or achalasia can also produce significant symptoms without a radiologic recurrence and needs to be considered and appropriately investigated.
Work-up for revisional surgery
A thorough work-up for reoperative surgery begins with a comprehensive history to understand timing, duration, type and severity of symptoms (Table 1). Obtaining the details of the initial surgery is crucial to understanding the anatomy and possible pitfalls leading to the recurrence or symptoms. Important factors to elucidate are extent of mediastinal dissection for esophageal mobilization, removal of sac, type of crural closure and length of intra-abdominal esophagus. In addition, details of esophageal lengthening procedure, wrap construction, potential injury to vagus nerves, and results of any intra-operative endoscopy are useful for planning and correlation with barium swallow images. Given that many of the revisional surgeries are performed by a surgeon different than the original surgeon, pre-operative work up can be a reasonable starting point. However, intra-operative exploration will often identify new findings not noted in the pre-operative work up. As such, the redo surgeon should be willing to adapt their operative strategy to address the unexpected (Table 2).
Table 1
| ❖ Detailed history (obstructive symptoms, gastroparesis) |
| ❖ Physical exam |
| ❖ Previous operative report details (use of mesh, esophageal lengthening procedure, wrap construction, preservation of vagus nerves) |
| ❖ Barium esophagogram |
| ❖ EGD |
| ❖ Gastric emptying study |
| ❖ Manometry |
| ❖ ± pH study |
EGD, esophagogastroduodenoscopy.
Table 2
| ❖ Incorrect/missed diagnosis |
| ⬥ e.g., missed achalasia with 360o wrap performed |
| ❖ Incorrect wrap construction |
| ⬥ Too tight, torsed, slipped, wrap on gastric body instead of fundus |
| ❖ Crural closure failure |
| ⬥ Hiatus too wide, peritoneum stripped weakening closure, crura on tension |
| ❖ Inadequate intra-abdominal esophagus |
| ⬥ Failure to perform adequate mediastinal mobilization of esophagus, no esophageal lengthening procedure completed |
| ❖ Incomplete removal of the GE fat pad |
| ⬥ Incorrect identification of GEJ, wrap is around GE fat pad rather than esophagus |
GE, gastroesophageal; GEJ, gastroesophageal junction.
Our usual investigations when considering a patient for re-operative surgery includes an esophagogastroduodenoscopy (EGD) by the operating surgeon to evaluate the wrap for potential torsion, slippage, and tightness. Esophagogram provides excellent anatomic and functional details and is at times supplemented by CT of the chest. Manometry is often performed especially if a missed diagnosis of esophageal dysmotility is suspected. In cases of large esophageal recurrences, positioning of manometry probes may be impossible and inaccurate. Gastric emptying studies are a part of routine work up to investigate any vagal injury and subsequent gastroparesis from the original surgery. We consider 24-h pH monitoring on an individual basis depending on gastroesophageal reflux disease (GERD) symptoms, especially so in those without endoscopic findings of reflux, or atypical GERD symptoms.
Approach to re-do surgery
Approaching reoperative paraesophageal hernia surgery needs a customized approach depending on the type of the index operation, any ensuing complications (e.g., leak), possibility of a hostile abdomen, and number of previous reoperations.
Transabdominal
Abdominal access is the most commonly performed approach for redo paraesophageal hernia repairs. Most commonly if the index operation was minimally invasive, the re-operation can also begin similarly with conversion to a laparotomy as required. The extent of the adhesions, timing of recurrence, size of the recurrent hernia and use of mesh to close the crura can affect the success of this approach. Extensive adhesions can be seen in prior open surgery and in cases where there may have been a peri-operative leak. While this is not a contraindication for abdominal approach (even minimally invasive approaches), this certainly tempers one’s enthusiasm to persist in this approach. Large recurrent hernias and hernias that may have recurred early may be adherent to mediastinal structures and limiting mobilization back into the abdominal cavity. Similarly, the use of mesh, especially prosthetic mesh, can create tremendous challenge in reducing the hernia through a trans abdominal approach. In the case of a large hernia and mesh, either a partial resection of the crura with mesh or leaving some mesh may be needed to successfully complete a redo repair. These scenarios may require a thoracic approach.
Thoracotomy
Access through a thoracotomy facilitates dissection in circumstances of a hostile abdomen with a herniated wrap. The posterolateral thoracotomy incision is typically in the left 7th intercostal space. Dissection begins with division of the inferior pulmonary ligament until the inferior pulmonary vein. The hernia sac is dissected away from the pericardium anteriorly and the aorta posteriorly. At this point the esophagus is encircled with a penrose. The dissection continues inferiorly until the right crus is identified and retracted. Any remaining hernia sac is opened thus entering the abdomen. The sac is opened circumferentially 1–2 cm away from the crus to preserve crural integrity. The Belsey artery located posteriorly needs to be cautiously avoided as injury can cause unrecognized bleeding into the abdomen. Any remaining hernia sac and gastroesophageal (GE) fat pad is resected with caution about the location of the vagii. The esophagus is assessed for adequate intra-abdominal length. Through a chest approach a generous mediastinal mobilization is possible which decreases need for esophageal lengthening. If necessary a Collis gastroplasty can be completed in the usual manner over a 50-F bougie. A Belsey mark IV, Toupet or Nissen fundoplication are then fashioned prior to crural closure.
Thoracoabdominal
A left thoracoabdominal access provides superior visualization in the abdomen and the chest compared with a thoracotomy and should be considered in cases with a hostile abdomen yet a need for intra-abdominal dissection. In circumstances of an infra-diaphragmatic wrap with herniated cardia or mesh with a hostile abdomen, a thoracoabdominal access can facilitate a safe and efficient dissection. Another option is division of the diaphragm through a thoracotomy however this potentially weakens an already weak diaphragm. The thoracoabdominal approach is also a versatile access when considering another operations on recurrent hernia such as Roux-en-Y gastric bypass (GB) or esophagectomy.
Although this formidable incision increases morbidity such as costal arch dehiscence, diaphragmatic hernia and wound complications, these risks can be minimized with experience and a meticulous closure technique (6).
Surgical considerations
We usually begin the surgery laparoscopically in cases where the prior surgeries are also laparoscopic with no reason for a frozen abdomen. The first port is an open Hasson supra-umbilical midline port using previous incision followed by insertion of other ports under direct vision. Right or left subcostal entry are other options. Remaining ports are placed based on surgeon preference of using a split table versus a standard supine position. Our general series of steps involves (I) survey and exposure, (II) crural dissection and mesh removal, (III) reducing the hernia, (IV) undoing the wrap, (V) performing gastroplasty if indicated, (VI) closure of the crura, and (VII) reconstruction of the wrap.
Survey and exposure
The first step towards exposure begins with liver retraction which may take significant adhesiolysis prior to insertion of a liver retractor. We prefer to do this with sharp dissection with great caution to avoid an enterotomy of the anterior wall of the stomach and the esophagus which is often adhered to the left lobe of the liver. Superficial liver tears can be managed with electrocautery and topical hemostatic agents. Unlike primary paraesophageal hernia repairs where the overwhelming majority are approached minimally invasively, revisional surgery may require open surgery based on intra-operative findings as well as surgeon preference.
Crural dissection and mesh removal
Dissection of the crura along with removal of any inserted mesh is a crucial step towards restoring normal anatomy. It can prove to be especially challenging with circumferential mesh insertion. The caudate lobe is a useful marker to locate the right crus of the diaphragm. Intra-operative endoscopy and bougie insertion are helpful adjuncts to meticulous sharp dissection. Bleeding during dissection should be carefully examined as it may occur due to entry into the crural fibers or the esophageal muscle wall and needs prompt re-direction. Restraint should be employed in using thermal devices so as to avoid delayed perforations. Integrity of the peritoneal lining on the crura needs to be preserved for strength during closure. If mesh is present we focus on dissecting out the parts required to complete crural dissection and restore anatomy. Unless it is infected, the entirety of the mesh does not need resection.
Reducing the hernia
Once crural dissection is complete, mediastinal dissection to reduce the hernia can proceed. The hernia sac, if remaining from the index operation, is grasped and the plane between the sac and the mediastinum developed. If no significant hernia sac remains, dissection is performed with extra precaution to avoid esophageal and stomach injury. Mediastinal dissection is often quite challenging in early recurrences as the gastric wall may be adherent to mediastinal structures such as the pericardium or lung. The tautness of the posterior vagus can often be palpated more easily than visualized during this part. The anterior vagus if identified should be carefully preserved.
Unraveling the previous wrap
Once the entire hernia is reduced, the anatomy of the wrap is examined. The wrap may be slipped, undone or too tight. If it is a Nissen fundoplication identifying and dissecting the point of junction between the two edges of the wrap is an important landmark. This can then be unfurled with an endoGIA stapler. The anterior vagus nerve is especially vulnerable during this dissection. The stomach should also be mobilized and any remaining short gastric vessels should be ligated if not previously done so. Posterior attachments to the pancreas are also taken down.
Gastroplasty
Once normal anatomy is restored, intrabodominal esophageal length is confirmed. We perform intra-operative endoscopy to identify the GEJ and confirm the preservation of the esophageal mucosa. A leak test is also completed with endoscopic insufflation and a surgical field filled with saline. Any remaining GE fat pad is removed. At least 2.5–3.0 cm of tension free intraabdominal esophagus is required, and if not present after mediastinal dissection a gastroplasty is fashioned. Gastroplasty can be performed via a wedge gastroplasty or through the chest with a single fire GIA over a 50-F bougie.
Closure of the crura
Once intra-abdominal length is established, crura is re-approximated over a 48–50 F bougie allowing for easy passage of a blunt instrument. Preservation of the peritoneal lining, results in secure closure. If tension is found, reduction of pneumoperitoneum or careful induction of pneumothorax are a few strategies. If tension persists then a relaxing incision on the diaphragm (with on the left or the right side) can be fashioned which is subsequently closed with permanent mesh. Our preference is to avoid a mesh as much as possible due to possibility of infection and erosion into the esophagus. There is no significant evidence to indicate that mesh repair decreases hiatal hernia recurrence (7).
Reconstruction of the wrap
The fundoplication planned needs to consider esophageal motility and the reasons for the re-operation (Figure 2). In absence of any dysmotility, we perform a floppy Nissen fundoplication. If dysmotility is suspected, a partial fundoplication such as Toupet is considered. We rarely perform a Dor unless there is a concurrent esophageal myotomy performed. Gastropexy may be considered in the elderly or emergency/torsion cases. Regardless of the type of wrap planned key tenets include a floppy wrap, use of the stomach fundus and not the body, and affixation to the esophageal wall (and not just remaining GE fat pad). Endoscopy is performed on completion to confirm that closure of hiatus is not too tight, fundoplication is not twisted and esophageal mucosa is intact.
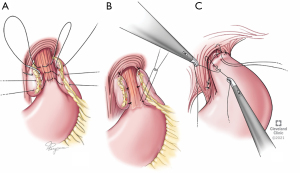
Intra-operative complications
Major intra-operative risks of re-do surgery include esophageal perforations, gastric perforations, vagii injury, and liver or spleen bleeding. A small esophageal injury, especially if occurring with sharp dissection, can be repaired in two layers in addition to a tissue buttress. A barium esophagogram testing is completed prior to resuming oral intake. Stomach injuries can either be repaired primarily or if the location permits, incorporated within a wrap or the wedge of a gastroplasty.
The anterior vagus is especially prone to injury during re-operations. It is imperative if the anterior vagus is already divided in the index operation that the posterior vagus is palpated or visualized and preserved with great care. When suspecting bilateral vagal injury or there is evidence of pre-operative gastroparesis, a drainage procedure such as pyloromyotomy or botox injection can be considered.
Bleeding from the liver especially during initial adhesiolysis is often self-limited. We ensure an excellent hemostasis before closure to prevent a compressing hematoma. Conversely, splenic injury occurring during either adhesiolysis, or division of the short gastrics, is more unforgiving. If local hemostastic maneuvers do not work, then a splenectomy is completed.
When to consider Roux-en-Y and esophagectomies
In the setting of intractable strictures, mesh erosion, severe dysmotility and multiple previous surgeries, an esophagectomy may be considered. Unsalvageable esophageal injuries may also require esophagectomy (7). Obese patients (especially BMI >35 kg/m2) with recurrent hernias should be considered for Roux-en-Y GB.
Post-operative management and complications
Patients are extubated in the operating room and recover in the post anesthesia care unit before transitioning to a regular thoracic surgery nursing floor. Avoiding nausea, retching or vomiting is key to preventing crural and wrap disruption and therefore regularly administered anti-emetics are crucial.
A nasogastric tube (NGT) is left for all reoperations and removed on the first sign of bowel function such as flatus or bowel movement which is typically on post-operative day (POD) 2. Upon removal of the NGT, a barium swallow is conducted to rule out leak and ensure smooth passage of contrast. We also leave a JP drain in the abdomen which is removed before dismissal. A full liquid diet is started after return of bowel function, and a soft anti-reflux diet for weeks 2–4 after which a gradual return to a normal diet is instituted.
On the first clinic visit typically 7–10 days from discharge, a chest X-ray is performed to rule out pleural effusions. Barium swallow and a 24-h pH study are performed at the 3-month follow up and then yearly for surveillance with imaging along with quality of life assessments.
Immediate post-operative complications are elevated in re-operative foregut surgery and include bleeding, splenic trauma, esophageal or gastric perforation and wound complications. Risk of vagal injury is certainly elevated due to distorted anatomy and dissection planes. Gastroparesis will become evident only in the post-operative recovery period and has required a gastric emptying procedure in up to 12% of patients who underwent revisional surgery (8). Some surgeons may choose a pyloromyotomy at time of re-operation if this is highly suspected or confirmed on pre-operative work-up; alternatively botox injection at the pylorus through an EGD is also option. Should Botox be used and symptoms of gastroparesis return, a per oral endoscopic pyloromyotomy (POP) may be performed. Temporary dysphagia may be encountered if there edema at the wrap site—EGD with gentle dilation can relieve this symptom until the edema subsides. Reflux and gas bloat require dietary changes and medical management.
Conclusions
Revisional surgery for paraesophageal hernia is fraught with a higher risk of complications. Although it has a decreased rate of symptom relief in comparison with first time operations, up to 80% of patients can experience symptom resolution or improvement. Careful and thorough work up is necessary to ensure anatomic and functional derangements are identified to plan the appropriate procedures at reoperation. Experienced foregut surgeons can approach most cases successfully with a minimally invasive approach, with alternatives of laparotomy, left thoracotomy and thoracoabdominal approach for more complex cases. Consideration to mesh extraction, reduction of hernia with complete resection of sac (if remaining), ensuring adequate intra-abdominal esophageal length, revising the fundoplication and appropriate closure of crura are key considerations. Minimizing risk of bilateral vagal injury, esophageal perforations and gastric perforations are paramount. Post-operative management will likely benefit from a conservative approach with NGT decompression in the immediate post-operative period followed by a barium esophagogram prior to diet advancement. Longitudinal follow up with barium esophagograms and quality of life assessments is essential in this patient population to identify sequelae of their redo surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Rishindra M. Reddy) for the series “Paraesophageal Hiatal Hernia Repairs, Transthoracic, Transabdominal, Laparoscopic, or Robotic, Which Method is Best” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://vats.amegroups.com/article/view/10.21037/vats-21-31/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-21-31/coif). The series “Paraesophageal Hiatal Hernia Repairs, Transthoracic, Transabdominal, Laparoscopic, or Robotic, Which Method is Best” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kao AM, Otero J, Schlosser KA, et al. One more time: redo paraesophageal hernia repair results in safe, durable outcomes compared with primary repairs. Am Surg 2018;84:1138-45. [Crossref] [PubMed]
- Munie S, Nasser H, Gould JC. Salvage options for fundoplication failure. Curr Gastroenterol Rep 2019;21:41. [Crossref] [PubMed]
- Koetje JH, Oor JE, Roks DJ, et al. Equal patient satisfaction, quality of life and objective recurrence rate after laparoscopic hiatal hernia repair with and without mesh. Surg Endosc 2017;31:3673-80. [Crossref] [PubMed]
- Furnée EJ, Draaisma WA, Broeders IA, et al. Surgical reintervention after failed antireflux surgery: a systematic review of the literature. J Gastrointest Surg 2009;13:1539-49. [Crossref] [PubMed]
- Suppiah A, Sirimanna P, Vivian SJ, et al. Temporal patterns of hiatus hernia recurrence and hiatal failure: quality of life and recurrence after revision surgery. Dis Esophagus 2017;30:1-8. [Crossref] [PubMed]
- Henderson RD, Marryatt G. Recurrent hiatal hernia: management by thoracoabdominal total fundoplication gastroplasty. Can J Surg 1981;24:151-3, 157. [PubMed]
- Shen KR, Harrison-Phipps KM, Cassivi SD, et al. Esophagectomy after anti-reflux surgery. J Thorac Cardiovasc Surg 2010;139:969-75. [Crossref] [PubMed]
- Hamrick MC, Davis SS, Chiruvella A, et al. Incidence of delayed gastric emptying associated with revisional laparoscopic paraesophageal hernia repair. J Gastrointest Surg 2013;17:213-7. [Crossref] [PubMed]
Cite this article as: Sudarshan M, Raja S. Re-operative surgery after paraesophageal hernia repair: narrative review. Video-assist Thorac Surg 2022;7:9.


