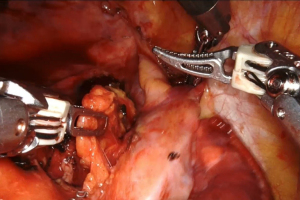
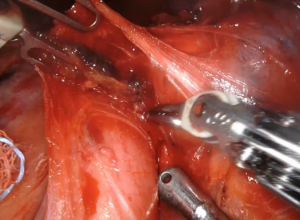
Robotic hilum-mediastinal lymph nodes dissection for operable non-small cell lung cancer (NSCLC) patients: state of art
Introduction
The standard surgical procedures for patients with early-stage non-small cell lung cancer (NSCLC) is lobectomy associated radical lymphadenectomy (1). Despite the thoracotomy approach is considered the gold standard, minimally invasive techniques have increasingly strengthened their role in lung cancer treatment with improved peri-operative outcomes (2). Lymph node dissection is a crucial component in the surgical treatment of NSCLC, indeed survival after surgery depends on the number of pathological lymph nodes (3). Therefore, lymph nodal upstaging can be considered a benchmark for surgical quality of the procedure. In the era of precision medicine, we should choose the surgical approach which leads to the best oncological results. Despite the minimally invasive lobectomy technique has been accepted, regarding the lymph nodes dissection. Although previous studies have shown that video-assisted thoracoscopic surgery (VATS) can yield an adequate lymph nodes dissection, other studies have observed that nodal upstaging with VATS was less prevalent compared to thoracotomy. A perceived benefit of robotic surgery is its ease of use for lymph node dissection; thus, an accurate histopathological evaluation of the hilum-mediastinal lymph nodes seems to be more feasible. In this review we would explore the technical aspects of the robotic lymph node dissection and discuss the state of art of this technique.
Technical aspects
For complete resection of NSCLC, a systematic nodal dissection is always recommended (4). A correct hilum and mediastinal lymphadenectomy in the early-stage NSCLC surgery is associated to an accurate staging, which further guides any potential adjuvant treatment. Although the European Society of Thoracic Surgeons (ESTS) and International Association for the Study of Lung Cancer (IASLC) guidelines recommended a systematic lymph nodes dissection (5,6), in a significant part of the patients undergoing lung cancer curative surgery is not performed in the routinely surgical activity (7). According to the IASLC guidelines, at least three mediastinal nodal stations (but always subcarinal) should be excised as a minimum requirement.
The three robotic arms must be used simultaneously in order to simplify the exposure of the anatomical limits and the structures that should be preserved. Dissection can be performed using the bipolar forceps, the bipolar dissector and the retractor forceps.
The excision of the hilar lymph nodes is carried out during the bronco-vascular dissection. The mediastinal lymph nodes dissection can be performed before the lobectomy in order to simplify the isolation of the bronco-vascular structures. The lymph nodes removed should be separately labeled and examined histologically. In general, to achieve a complete removal, all the mediastinal fat tissue around the lymph nodes should be dissected within anatomical landmarks. After pathological examination of the lymph nodes specimens, the number of the involved lymph nodes, the nodal stations, and the status of the nodal capsule should be documented.
Patient positioning and port placement
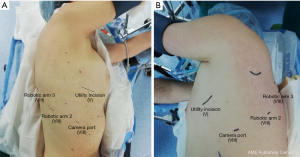
The operations were performed in the lateral decubitus and under general anesthesia using a double-lumen tube with single-lung ventilation. The da Vinci system is placed behind the patient. First, we performed a 3-cm anterolateral utility incision, usually at the 5th or 6th intercostal space saving the latissimus dorsi and splitting the serratus anterior along its muscle fibers. The wound is protected using a soft tissue retractor. Through a 30-degree robotic camera, we performed the other 8-mm three ports usually at the 8th or 9th intercostal space and we carried out the robot docking. Then, the bed-assistant introduced the operative robotics instruments (Figure 1).
Left side
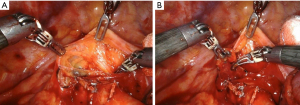
The lymph nodes dissection in the left side of the chest should be performed starting from the opening of the mediastinal pleura posteriorly and anteriorly at the hilum. Therefore, the main pulmonary artery and the superior pulmonary vein must be exposed. The dissection should be conducted following the phrenic nerve along the pericardium. During the dissection within the limits of the aortopulmonary window, attention should be done to the recurrent laryngeal nerve and to the vagus nerve that must be isolated and retracted with the robotic arm. The mediastinal lymph nodes stations that are minimally required are the subaortic (station 5), para-aortic (station 6) and inferior paratracheal (4L). To access these stations, the left lung should be retracted inferiorly in order to expose the aortopulmonary window (Figure 2). This step can be carried out by the assistant while the surgeon can use the three arms for the dissection.
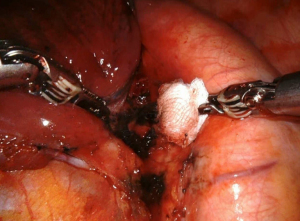
The upper border of station 5 is the lower border of the aortic arch and the lower border is the upper rim of the left main pulmonary artery. Paraaortic lymph nodes are anteriorly and laterally to the ascending aorta and the aortic arch. The station 6 is between the upper border of the aortic and the lower border of the aortic arch. The upper border of station 4L is the upper margin of the aortic arch. The lower border is the upper rim of the left main pulmonary artery. The left lateral border of the trachea is considered the anatomical limit between the station 4R and 4L.
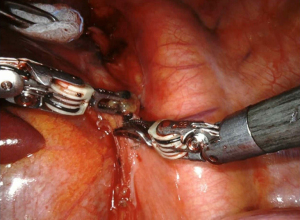
The subcarinal space (station 7) can be accessed by retracting the lung medially (Figure 3). The aortic arch on the left side of the chest makes the resection of this station more challenging. The dissection should be conducted between the carina of the trachea and the upper border of the lower lobe bronchus. If the dissection of this station is performed after a left lower lobectomy, with the robotic arm the bronchus stamp must be retracted anteriorly. The dissection of the subcarinal tissue should be performed until the exposure of the contralateral bronchus. After the division of the pulmonary ligament, the levels 8 and 9 lymph nodes are completed (Figure 4).
Right side
The procedures in the right side should be started opening the paratracheal area and the lung must be retracted inferiorly. The assistant can help the surgeon on the retraction of the lung. The tissue of the station 2R and 4R should be resected ideally all at once (Figure 5). The mediastinal pleura is opened above the upper margin of the azygos vein The limits of the removal are: cranially, brachiocephalic trunk; medially, the ascending aorta and origin of aortic arch; anteriorly, the superior vena cava; posteriorly, the esophagus; and inferiorly, the pulmonary artery. If an additional exposure of the paratracheal space is needed, the azygos vein can be divided. The vagus nerve must be isolated and retracted anteriorly in order to avoid injuries. The three robotic arms make easier the preservation of the nerves. During the dissection of the paratracheal space a small venous branch that empties into the superior vena cava can be found and should be clipped or divided using the bipolar dissector. More challenging is the dissection of the station 3a and 3p but in case of visible nodes in front of the superior vena cava and posterior to the trachea resection should be done. The dissection of the inferior mediastinal right stations usually starts after the division of the inferior pulmonary ligament and the resection of the station 9. After the isolation of the inferior pulmonary vein, the paraoesophageal lymph nodes (station 8) are resected, taking care to the preservation of the vagus nerve. The dissection is conducted medially to the vagus nerve that is retracted using the robotic forceps. As the left side of the lung, the subcarinal space is accessed by retracting the lung medially (Figure 6). The dissection following the right main bronchus and the resection should be performed until the exposure the contralateral main bronchus. The resection of this station can become challenging for the bleeding from the raw surface of the lymph nodes that can be controlled with the two bipolar arms and the complete removal of all the nodal tissue. In order to avoid the rupture of the lymph node during the dissection the traction should be done taking with the robotic grasper the tissue around the lymph node. Care must be done to the bronchial artery that are in this area.
Discussion
Although the preoperative staging techniques are strongly improved, the number of hidden positive lymph nodes is still high (8). Therefore, a correct lymphadenectomy should be one of the main goals during the lung cancer surgery (9). Survival following surgery for node-negative NSCLC is associated with the number of lymph nodes analyzed (10). A higher numbers of lymph nodes resected allow a better staging and reduce the risk of missing positive lymph nodes.
In the last few years, the minimally invasive techniques were strongly improved gaining prominence in the daily surgical activity. First minimally invasive technique used was the VATS with excellent morbidity and mortality outcomes (11). A fewer complications rate, less postoperative pain and shorter hospital stay are the main short-term results of the studies that compared the VATS procedures to thoracotomy (12). The long-term efficacy of VATS for lung cancer surgery is still uncertain. The controversy is represented by the nodal upstaging rate compared to the gold standard thoracotomy. One of the first study that compared the VATS and open lobectomy plus radical lymphadenectomy was conducted by Licht et al. evaluating the short- and long-term outcomes of 1,513 lobectomies for stage I NSCLC. The VATS group showed a lower upstaging rate compared to thoracotomy group but no differences in overall survival were found (13). Boffa et al. reported a similar nodal upstaging rate between the VATS group and the thoracotomy group in a cohort of 11,500 patients from the Society of Thoracic Surgeon database (14). Difference was found in terms of number of lymph nodes dissected on the mediastinal stations between the open group and the VATS, probably due to the more challenging dissection for a limited angle of maneuverability of the thoracoscopic instruments. In the last years the robotic surgery has become part of the routine of many surgical specialties (15). The robotic approach constitutes a technological development of the VATS procedure. This is due to the technical advantages such as: a better view of the operative field (3D instead of 2D), an easier use of the instruments that are more comfortable for the surgeon, more precise movements related to the wide angle of maneuverability of the instruments, which is even superior to that of the human hand (16).
For the mediastinal lymphadenectomy, the three operative arms allow to achieve an excellent view of the anatomical limits. The exposure of the anatomical structures that should be preserved during the resection is allowed by the 3D viewing of the camera. For example, the right paratracheal area can be accurately resected exposing the trachea, preserving the vagus nerve and respecting the limits of the superior vena cava, the azygos vein and the brachiocephalic trunk. The aortopulmonary window can be accurately explored avoiding the lesions of laryngeal nerve. The three arms allow the right tractions to achieve a better dissection of station 7 from both the sides until the contralateral principal bronchus. The robotic technology provides an easier standardization of these steps and the dissection can be performed safely. The bleeding is the first complication of the mediastinal lymph nodes dissection and in case of deep surgical areas the hemostasis can be challenging. The accuracy of the robotic arms allows an excellent control of the bleeding and using simultaneously the bipolar forceps and the bipolar dissector, the hemostasis can be easily carried out. In the last few years, the analysis of lymph nodes dissection in NSCLC patients undergoing radical surgical treatment is a debated issue (17). The first multicentric retrospective upstaging analysis in patients with clinical stage I NSCLC, which underwent robotic anatomical resections was reported by Wilson. The nodal upstaging was observed in 10.9% of cases, especially in those patients with larger lung tumor (18). Zirafa et al. in a retrospective study that compared the lobectomy performed with the robotic approach to the thoracotomy reported a higher upstaging rate in terms of the N2 disease in the robotic group (19). In the last years, some studies were done about the cost of the robotic surgery. The increased cost of robotic surgery could be a problem and may slow its use in the daily surgical practice. However, Novellis et al. reported that the robotic approach was profitable for the hospital in that it cost about 18% less than the current health service reimbursement (20). These results were confirmed by other studies that analyzed the cost of the procedures (21,22).
Others studies should be conducted to identify what is the better minimally invasive procedures that can be compared with thoracotomy but the previous results showed that the robotic mediastinal lymph nodes dissection can be carried out safely and leads a better pathological staging of the disease.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Video-Assisted Thoracic Surgery for the series “Lymphadenectomy during VATS and RATS: state of the art”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-21-18/coif). The series “Lymphadenectomy during VATS and RATS: state of the art” was commissioned by the editorial office without any funding or sponsorship. FF served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. [Crossref] [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Rami-Porta R, Wittekind C, Goldstraw P, et al. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25-33. [Crossref] [PubMed]
- Verhagen AF, Schoenmakers MC, Barendregt W, et al. Completeness of lung cancer surgery: is mediastinal dissection common practice? Eur J Cardiothorac Surg 2012;41:834-8. [Crossref] [PubMed]
- Edwards JG, Chansky K, Van Schil P, et al. The IASLC Lung Cancer Staging Project: Analysis of Resection Margin Status and Proposals for Residual Tumor Descriptors for Non-Small Cell Lung Cancer. J Thorac Oncol 2020;15:344-59. [Crossref] [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- van der Woude L, Wouters MWJM, Hartemink KJ, et al. Completeness of lymph node dissection in patients undergoing minimally invasive- or open surgery for non-small cell lung cancer: A nationwide study. Eur J Surg Oncol 2021;47:1784-90. [Crossref] [PubMed]
- Al-Sarraf N, Aziz R, Gately K, et al. Pattern and predictors of occult mediastinal lymph node involvement in non-small cell lung cancer patients with negative mediastinal uptake on positron emission tomography. Eur J Cardiothorac Surg 2008;33:104-9. [Crossref] [PubMed]
- Chiappetta M, Leuzzi G, Sperduti I, et al. Lymph-node ratio predicts survival among the different stages of non-small-cell lung cancer: a multicentre analysis†. Eur J Cardiothorac Surg 2019;55:405-12. [Crossref] [PubMed]
- Bille A, Woo KM, Ahmad U, et al. Incidence of occult pN2 disease following resection and mediastinal lymph node dissection in clinical stage I lung cancer patients. Eur J Cardiothorac Surg 2017;51:674-9. [Crossref] [PubMed]
- Yan TD, Cao C, D'Amico TA, et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg 2014;45:633-9. [Crossref] [PubMed]
- Scarci M, Pardolesi A, Caruana EJ, et al. Video-assisted thoracoscopic lobectomy: operative technique. Multimed Man Cardiothorac Surg 2015;2015:mmv014. [Crossref] [PubMed]
- Licht PB, Jørgensen OD, Ladegaard L, et al. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg 2013;96:943-9; discussion 949-50. [Crossref] [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-53; discussion 353. [Crossref] [PubMed]
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. [Crossref] [PubMed]
- Chiappetta M, Leuzzi G, Sperduti I, et al. Mediastinal Up-Staging During Surgery in Non-Small-Cell Lung Cancer: Which Mediastinal Lymph Node Metastasis Patterns Better Predict The Outcome? A Multicenter Analysis. Clin Lung Cancer 2020;21:464-471.e1. [Crossref] [PubMed]
- Wilson JL, Louie BE, Cerfolio RJ, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg 2014;97:1901-6; discussion 1906-7. [Crossref] [PubMed]
- Zirafa C, Aprile V, Ricciardi S, et al. Nodal upstaging evaluation in NSCLC patients treated by robotic lobectomy. Surg Endosc 2019;33:153-8. [Crossref] [PubMed]
- Novellis P, Bottoni E, Voulaz E, et al. Robotic surgery, video-assisted thoracic surgery, and open surgery for early stage lung cancer: comparison of costs and outcomes at a single institute. J Thorac Dis 2018;10:790-8. [Crossref] [PubMed]
- Nasir BS, Bryant AS, Minnich DJ, et al. Performing robotic lobectomy and segmentectomy: cost, profitability, and outcomes. Ann Thorac Surg 2014;98:203-8; discussion 208-9. [Crossref] [PubMed]
- Paul S, Jalbert J, Isaacs AJ, et al. Comparative effectiveness of robotic-assisted vs thoracoscopic lobectomy. Chest 2014;146:1505-12. [Crossref] [PubMed]
Cite this article as: Gallina FT, Alessandrini G, Facciolo F. Robotic hilum-mediastinal lymph nodes dissection for operable non-small cell lung cancer (NSCLC) patients: state of art. Video-assist Thorac Surg 2022;7:3.