
VATS mediastinal lymph node dissection: surgical technique and literature review
Introduction
Roviaro et al. performing the first video-assisted thoracoscopic surgery (VATS) lobectomy more than 20 years ago (1), started a new era in lung cancer surgery. The better outcomes compared to “open procedures” in terms of less pain, fewer post-operative complications, reduced chest drainage duration and shorter length of stay has, in fact, prompted nearly every thoracic surgeon in the world to at least attempt to VATS approach.
The hypothetical differences in terms of local recurrences and long-term survival of VATS approaches compared with open procedures have been overcome (2) and it is proved by then that VATS lobectomy can offer, if performed by skilled surgeons, a better complications rate and the same safety profile of open surgery.
Since 2014 we routinely use the biportal VATS technique for lobectomy and, exceptionally, pneumonectomy (3) believing that it is safe and effective. We have proved, in fact, that a biportal VATS (camera-port with a single utility incision) leads to a lower length of hospitalization (P<0.05), lower duration of chest tube (P<0.05) and less pain (P<0.05) compared with three- or four-ports groups (4). Some tricks are to be considered like the sharp separation by scalpel instead of monopolar incision and the carefully protection of phrenic and laryngeal nerves during the dissection of mediastinal lymph nodes (right side: station 2, 3, 4, 7, 8, 9; left side: station 4, 5, 6, 7, 8, 9).
Pulmonary lobectomy is the standard surgical treatment of early-stage lung cancer, only if it is associated with mediastinal lymph node dissection (MLND) (5,6). Lymph node dissection, detecting clinically occult metastases, can control local tumor growth reducing effectively local recurrence. Furthermore, upstaging the nodal status of the patients due to its sensitivity and specificity enables the correct use of adjuvant therapy and improve the long-term survival.
If there is a consensus on the downstaging rate of non-invasive examinations such as chest CT and PET/CT as suggested by the CALGB 9761 prospective trial (7) there is, otherwise, controversial whether VATS approach can achieve the same quality of an open approach in MLND.
Starting with the description of our experience in multi-portal VATS lobectomy we present the different lymph node dissection techniques starting from the coded and most used ones. Then, we add our tricks and tips that allow us to achieve the results presented. We also discuss about the accuracy of this procedure reviewing the data of the most authoritative literature on this topic.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://vats.amegroups.com/article/view/10.21037/vats-21-27/rc).
Methods
Since their introduction VATS procedures have gained more and more importance until now when at our Institution they are applied, compared with open procedure, with a ratio of more than 85% vs. 15%. The reasons of their widespread use are to be found in the decrease of post-operative pain, length of stay, duration of chest tube and costs of hospitalization that minimally invasive thoracic surgery can guarantee.
From May 2012, date of starting of VATS surgery program at Vito Fazzi Hospital - Lecce, to December 2020 we performed 42 procedures with a 4-port approach, 56 with a 3-port approach and 427 with a 2-port approach. Our surgical approach was ever focused on the most accurate dissection of pulmonary and mediastinal structures and on the best oncologic radical outcome. Since 2019 we perform, sometimes, also uniportal VATS for lobectomy (Table 1).
Table 1
| Characteristics | Value |
|---|---|
| Overall (n) | 525 |
| Gender (female), n (%) | 151 (28.8) |
| Age (years), mean (SD) | 69.0 (8.2) |
| Smoke, n (%) | 409 (78.2) |
| Comorbidity, n (%) | |
| Heart | 347 (66.1) |
| Liver | 16 (3.0) |
| Kidney | 15 (2.9) |
| Vascular | 126 (24.0) |
| VC%, mean (SD) | 91.2 (18.2) |
| FeV1%, mean (SD) | 87.8 (18.6) |
| pCO2 (mmHg), mean (SD) | 39.1 (6.2) |
| Type of surgical intervention, n (%) | |
| RUL/RML | 200 (38.1) |
| RLL | 117 (22.3) |
| LUL | 120 (22.9) |
| LLL | 88 (16.8) |
| Surgical time (min), median [IQR] | 120 [100–130] |
| Lymph nodes, mean (SD) | 7.82 (6.07) |
| pStage, n (%) | |
| IA | 409 (77.9) |
| IB | 59 (11.2) |
| IIA | 18 (3.4) |
| IIB | 12 (2.3) |
| IIIA | 23 (4.4) |
| IV | 4 (0.8) |
| Length of hospital stay (days), median [IQR] | 5 [5–6] |
| Complication, n (%) | 72 (13.7) |
| Recurrent laryngeal nerve | 9 (12.5) |
| Bronchial fistula | 1 (1.4) |
| Air leaks (>7 days) | 31 (43.1) |
| Atrial fibrillation | 6 (8.3) |
| Fluid retention | 11 (15.3) |
| Surgical reintervention | 9 (12.5) |
| Others | 5 (6.9) |
RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; IQR, interquartile range.
Starting with a review of the preeminent literature we reported our data in terms of LNs retrieved, number of LNs in each station N1 and N2 by side and by type of surgical intervention.
The accuracy of MLND could be analyzed in three different ways according with literature: (I) number of lymph node dissected (8); (II) rate of postoperative nodal upstaging (9,10); and (III) the weight of nodes dissected (8).
We analyzed the accuracy of MLND calculating and comparing the number of LNs dissected and rate of post-operative nodal upstaging due to the lack of data regarding their weight.
The RUL and RML interventions are considered together to be able to extend the comparison with the data of another author.
Furthermore, we investigated the presence of predictors associated with post-operative complications and length of hospitalization with particular interest for the number of lymph nodes removed. The same was done for the relationship between the number of LNs removed and the surgical time taking in account and reporting the short-term outcome in terms of post-operative complications.
Surgical technique of VATS lobectomy
The preparation of the patient begins with the positioning of a double-lumen endotracheal tube by the anesthesiologist. That tube is inserted under fiber bronchoscopy guidance during general anesthesia.
There is a growing consensus on the usefulness of non-intubated anesthesia. The awake thoracic surgery via VATS has several pros such as avoiding the need for general anesthesia, permitting a more physiological cardiac, pulmonary, and neurological state during the surgical procedure, and limiting postoperative nausea and vomiting. However, we believe that centers starting with such a procedure should begin by performing minor VATS procedures in selected low-risk patients. An early conversion is necessary for any situation of sudden surgical difficulty or cardiopulmonary instability. Then, we need to practice it even more with minor surgical interventions (11). For these reasons, we have not considered the surgical interventions performed by awake in the surgical series reported in this study.
The patient is then positioned in full lateral decubitus (clasp-knife position) flexing slightly the middle chest to allow splaying of the ribs.
VATS lobectomy requires total lung collapse and two incisions in the case of biportal VATS. The camera port, 1.5 cm sized, is placed at the level of 8th–9th intercostal space and the utility incision port, 4–5 cm sized, at 4th–5th intercostal space.
The dissection of vein, bronchus, and artery with lymph node dissection is performed individually. The instruments should be long and curved for their simultaneous insertion (two or three). Thus, we would rather a combination of traditional and thoracoscopic ones.
A 12-mm trocar for the 10 mm–30° camera is inserted through the lower incision. We usually start inspecting first the pleural cavity to exclude the presence of metastasis or hilar invasion. Secondly, we cut eventually adhesions especially in the case of the redo interventions.
The pulmonary vein is isolated and ligated anterior to the hilum or the lower pulmonary ligament. Subsequently, the bronchial branch and the artery were addressed using a stapler. In the end, in the case of an incomplete fissure, it is stapled. We describe our lobe specific technique of lobectomy as follows.
In the case of a right upper lobectomy, we dissect and suture first the upper pulmonary vein. Before to do that, it is very important to verify the anatomy of the middle lobar vein. It follows the suture of the Boyden truncus (apical-anterior branch of the upper pulmonary artery), that could be also proximally bi- or tri-furcated. It must be careful of the superior mediastinal artery to the upper lobe to avoid his rupture. It is a small artery usually lying under the posterior wall of the pulmonary vein and running toward the upper lobar bronchus. It could be sutured by a clip or hem-o-lok®. Dissecting the mediastinal pleura below the posterior wall of the bronchus and identifying the secondary carina could be useful to approach the upper lobar one. When dividing the fissures, the anvil of the stapler must be positioned over the hilum, taking care of the vascular elements and the bronchus already sutured (12).
To perform a middle lobectomy, we isolated and dissect the hilum elements as follow: vein, bronchus, and artery. After suture of the main artery behind the bronchus, it must pay attention to a second middle artery, inconstant according with our experience, originating from the intermediate artery. The middle lobe artery should be transected to allow us the dissection of the intermediate artery and to divide the fissure. This is particularly true when the fissure is not complete.
When performing a right lower lobectomy, the incision of the pulmonary ligament is mandatory to identify the lower lobar vein. During dissection of the right lower vein, we must take care of the middle vein, intrafissural dissection of the lower lobar and A6 artery.
During a left sided upper lobectomy, we approach first the upper lobar vein. There can be up to five arteries for the left upper lobe, originating from the mediastinal and fissural sides. If the fissure is incomplete, the upper lobe bronchus is detached from the main pulmonary artery (MPA) and then isolated and transected after the division of the mediastinal branches of pulmonary artery. Finally, the remaining branches of pulmonary artery come into view in the fissure and can be sutured (13,14). The fissure is then divided with a stapler. Sometimes the lingular artery lies just behind the upper lobar bronchus and it may be useful to carefully transect first the bronchus. Early confluence of the upper and lower veins is common.
Finally, we describe the left lower lobectomy that we consider as the simplest video-assisted thoracoscopic lobectomy. The incision of the pulmonary ligament helps us to identify the lower lobar vein. If the fissure is incomplete, the pleura, fat and lymph nodes between the veins are removed, exposing the main and lower lobe bronchus. Pushing up the parenchyma over the artery creates a plane between the upper wall of the artery and the parenchyma where the anvil of the stapler can be placed to divide the fissure. Alternatively, the mediastinal pleura is opened and the pulmonary artery is dissect until it enters the posterior part of the fissure. The lower pulmonary vein is dissected first, and then the lobar bronchus and the basal branch of the pulmonary artery that lies just over the bronchus. In the end, the fissure is transected.
As it is written above, the surgical instruments could be swapped from an incision to another. It can be important especially for upper lobectomies.
Starting the dissection of mediastinal structures performing the MLND could help the following phases of the surgical intervention. We must take in mind that the extent of the dissection should be not inferior to conventional thoracotomy as better described below.
At the end of the operation one 28-Fr intrathoracic drainage tube is inserted connected with a classical “bubble-in-chamber” drainage system or a digital chest drainage system.
Preoperative and intraoperative nodal staging
The mediastinoscopy is the gold standard for pre-operative (or primary) staging for patients with potentially operable lung cancer.
The American College of Chest Physicians practice guidelines could accept that invasive staging is probably not needed in those patients with peripheral tumors and no nodal involvement on computed tomography (CT) or positron emission tomography (PET) (15).
The European Society of Thoracic Surgeons guidelines confirm this position omitting the invasive staging for patients with stage I lung cancer and negative mediastinal PET imaging on the condition that the tumor is peripheral because of the high negative predictive value (NPV) of PET scan (16).
However, preoperative invasive nodal staging remains indicated in the case of central tumors, PET hilar N1 disease, low uptake at 18F-FDG PET scan of the primary tumor and large lymph-nodes on CT scan (≥16 mm).
The minimally invasive techniques (TBNA, EBUS-FNA, EUS-FNA) can be complementary to surgical invasive staging techniques because their specificity is high, but NPV is low.
Due to the non-negligible rate of unexpected pN2 disease in cN0–1 diseases during video-assisted major pulmonary resection as reported by many authors (17-19), MLND should be performed routinely intraoperatively even when nodal metastases are considered unlikely.
We can conclude that surgical mediastinal node staging is mandatory in every case of non-small cell lung cancer (NSCLC) operated because of major accuracy of VATS MLND than PET in staging the mediastinum.
Surgical technique of VATS MLND
After the description in 1956, by Nohl (20), of the lobe-specific lymphatic pathway Naruke proposed in 1978 an anatomical map where the lymph nodes grouped in stations were numbered. Stations 1 to 9 designate mediastinal N2 stations and stations 10 to 14 refer to N1 stations (21). On the same route went Mountain and Dresler (22) in 1997 publishing a new map of mediastinal lymph node stations. With these two maps was uniformed the recording of the lymph nodes involved and dissected during lung cancer surgery even if each of them has pro and cons (23).
We used the 8th edition of TNM Classification of NSCLC (24) and the lymph node map proposed by the International Association for the Study of Lung Cancer (IASLC) in 2009 (25). This map was proposed by IASLC to reconcile the differences between the Naruke and the Mountain and Dresler-ATS maps and to redefine the definitions of the anatomical boundaries of each lymph node station. It was incorporated into the seventh edition of the tumor, node, metastasis (TNM) staging system for lung cancer, published by the International Union Against Cancer (UICC) and the American Joint Committee on Cancer (AJCC).
Several techniques for VATS MLND have been reported and they could be schematically classified in grasping and non-grasping techniques (26,27).
The “grasping” technique is performed using a grasper or retractor that permits to grasp the target lymph nodes directly. The “non-grasping” technique, otherwise, essentially consists in a MLND using only energy devices (electrocoagulation hook or an ultrasound scalpel) and a metal endoscopic suction. Guo et al. have proved that this technique is superior to the traditional “grasping” technique (27). We use instead a sort of “hybrid node dissection technique” (4,28) with a massive use of energy devices, above all ultrasound based (e.g., Harmonic®, Sonicision®), that permits us to respect the anatomical boundaries well described by Isaka (29) and the node’s integrity. However, ligation of the connective tissue possibly with the minor lymphatic vessels can prevent post-operative complications such as chylothorax.
Our MLND’s technique, avoiding damaging the lymph nodes, helps us also to easily count the number of nodes retrieved.
As Watanabe et al. suggested (30) we think that to perform an oncological correct mediastinal node dissection two points should be considered mainly: (I) obtaining an excellent thoracoscopic view; (II) recognizing the boundary planes between the resected soft tissues and preserved organs (Table 2).
Table 2
| Lymph node compartments | Lymph node stations | Anatomical boundaries |
|---|---|---|
| Superior mediastinum | 2R | • Superior: apex of lung, thoracic inlet |
| • Inferior: intersection of caudal margin of the left BCA with the Tr | ||
| • Left: left (2L) and right (2R) are divided along the midline of the Tr | ||
| • Right: mediastinal pleura | ||
| • Anterior: PW of SVC | ||
| • Posterior: PW of Tr | ||
| 4R | • Superior: intersection of caudal margin of the left BCA with the Tr | |
| • Inferior: inferior border of the AV | ||
| • Left: left (4L) and right (4R) are divided along the midline of the Tr | ||
| • Right: mediastinal pleura | ||
| • Anterior: PW of SVC | ||
| • Posterior: PW of Tr | ||
| 4L | • Superior: superior border of the AA | |
| • Inferior: carina | ||
| • Left: medial to BL | ||
| • Right: left (4L) and right (4R) are divided along the midline of the Tr | ||
| • Anterior: AW of Tr | ||
| • Posterior: PW of Tr | ||
| Subaortic compartment | 5 | • Superior: inferior border of AA |
| • Inferior: superior border of left MPA | ||
| • Left: mediastinal pleura | ||
| • Right: plane between BL and left VN | ||
| • Anterior: left PN | ||
| • Posterior: left VN | ||
| 6 | • Superior: line tangential to the upper border of the AA | |
| • Inferior: lower border of the AA | ||
| • Left: mediastinal pleura | ||
| • Right: left LW of Asc Ao | ||
| • Anterior: AW of Asc Ao | ||
| • Posterior: PW of Asc Ao | ||
| Subcarinal and inferior compartment |
7 | • Superior: carina |
| • Inferior: SW of LUPV | ||
| • Left: MW of left main bronchus | ||
| • Right: MW of right main bronchus | ||
| • Anterior: pericardium | ||
| • Posterior: esophagus | ||
| 8 | • Superior: upper border of lower lobe bronchus on left, and lower border of bronchus intermedius on right | |
| • Inferior: diaphragm | ||
| 9 (lying within the pulmonary ligament) | • Superior: inferior pulmonary vein | |
| • Inferior: diaphragm |
MLND, mediastinal lymph node dissection; PW, posterior wall; AW, anterior wall; LW, lateral wall; SW, superior wall; MW, mediastinal wall; BCA, brachiocephalic artery; SVC, superior vena cava; Tr, trachea; AV, azygos vein; AA, aortic arch; BL, Botallo’s ligamentum arteriosum; PN, phrenic nerve; VN, vagus nerve; MPA, main pulmonary artery; Asc Ao, ascending aorta; LUPV, left upper pulmonary vein.
Three lymph node compartments can be explored and dissected: (I) the superior mediastinum especially in a right intervention (stations 2R, 4R, 4L); (II) the subaortic compartment in a left intervention (stations 5 and 6) and (III) the subcarinal (station 7) and inferior mediastinum in either a right- or left-sided approach (stations 7–14) (31).
The superior mediastinal compartment is that comprised, on the right side, between the posterior wall (PW) of superior vena cava and the PW of the trachea. Inferiorly there is the MPA and superiorly the right subclavian artery. This area comprises stations 2R and 4R.
On the left side, the anatomical boundaries are the anterior wall (AW) and PW of the trachea on a ventral-dorsal plane. We found, instead, the aortic arch and MPA superiorly and inferiorly respectively.
The subaortic compartment, containing station 5 and 6 (with interposition of the Botallo’s ligamentum arteriosum) is that comprises between the left phrenic nerve anteriorly and the left vagus nerve posteriorly. Superiorly we found the aortic arch and inferiorly the left MPA.
Finally, the subcarinal region (station 7) is well circumscribed anteriorly by the pericardium and PW of MPA, posteriorly by the mediastinal pleura and esophagus, superiorly by the carina and by the superior wall (SW) of the left upper pulmonary vein.
Following, we describe the surgical technique of VATS MLND separately as right and left MLND.
Right MLND
The pleura overlying the mediastinal stations 2R/4R is divided between the azygos vein and right brachiocephalic artery using electrocoagulation hook or an ultrasonic scalpel (e.g., Harmonic®, Sonicision®). Alternatively, we use a vessel-sealing system (e.g., Ligasure®). The azygos vein is needed to be divided rarely. The nodes with surrounded soft tissue are dissected en bloc from the anterolateral side of the trachea just below the caudal edge of the right brachiocephalic artery. The right vagus nerve is retracted backward with a peanut dissector. Then the lymph nodes block is dissected along the posterior wall of the superior vena cava (retracted anteriorly with a peanut dissector). Finally, the upper edge of the right MPA is dissected and the nodal block is removed under the azygos vein cranially.
According with our experience, and in contrast with other Authors, the dissection of the subcarinal nodes (station 7) is performed before the right main bronchus or right intermediate bronchus is taped. The carina is pulled into the operative field, which clearly exposes the left main bronchus. Exposure of the left main bronchus gives us the certainty that carinal station is completely explored. Lung tissue to be preserved is pushed and retracted toward the anterolateral side with a peanut dissector.
Lymph nodes could be absent at station 3 (station prevascular and retrotracheal), station 8 (paraesophageal station), and station 9 (pulmonary ligament station). We explore station 8 when we dissect station 7. Dissection of station 9 is performed when the inferior pulmonary ligament is excised.
Finally, dissection of N1 stations (e.g., stations 10, 11 and 12) is done when isolating contemporary pulmonary vein and artery, respectively.
Left MLND
The pleura is divided alongside the left vagus nerve. The pleura is divided from the upper edge of the left lower pulmonary vein to the lower edge of the aortic arch along the vagus nerve and the preserved lung is pushed. As previously reported, the branches of the left vagus nerve are divided from the bronchus.
The dissection of the station 4L is very difficult especially via VATS. The risk of damaging the laryngeal recurrent nerve is very high then it is needed to pay attention to this nerve especially when using energy devices. As suggested also by Nagashima et al. (32), one way that we are used to is to identify and transect the cardiac branch of left vagus nerve. Subsequently we exert a weak traction on it with a silk tread. The tension applied on the recurrent laryngeal nerve allows us to better visualize and dissect lymph node at station 4L. The left vagus nerve is pushed to the posterolateral side with a peanut dissector and the left-sided rotation of the surgical table could be useful.
Subcarinal nodes are often excised before the section of the bronchus. Thus, we do not use to traction the bronchial stump to expose the station 7. Gaining more and more experience, we have understood that could be useful to use a right single lumen tube when performing left lobectomy. This allows us to have a left main bronchus more mobile and therefore easier to expose the subcarinal station only with the help of the right lung ventilation increasing slightly PEEP. Instruments should be long and curved to permit the insertion of two or three instruments simultaneously. Then, we use a combination of traditional and thoracoscopic equipment. It is important to consider that to make an anatomical operation though two incisions, the camera and the instruments are exchanged from one incision to the other. Nagashima et al. suggests also to traction the main bronchus to gain a better view (32). The nodal station sited over the posterior pericardium is excised from the left lower pulmonary vein to the tracheal carina. Then, the nodal tissue is dissected alongside the right main bronchus. For this purpose, the esophagus is pushed with a peanut dissector and the nodal block is removed.
Sometimes we adopt for the MLND in station 7 the so called “anterior technique” described by Baste et al. (33). In order to open and dissect the subcarinal space the bronchial stump is lifted after the lobectomy by its anterior face.
The energy-based devices are very useful in pleura and lymph node dissection (34). The pleura overlying the mediastinal LN stations is divided using electrocoagulation hook or an ultrasonic scalpel (e.g., Harmonic®, Sonicision®). Alternatively, we use a vessel-sealing system (e.g., Ligasure®). Care must be taken using ultrasonic devices not to put the uncovered tip in contact with a pulmonary vessel or a nerve. For this reason, we would rather Harmonic® and Sonicision® over Ligasure® when performing MLNS at station 7. Otherwise, we prefer to use Ligasure® over Harmonic® and Sonicision® when performing MLNS at station 5 and 6.
To sum up, we should take in mind two important issues. Firstly, we must recognize the anatomical landmarks and dissect the mediastinal sheets as previously described. The nodal compartment should be completely free of lymph node and the anatomical structures well recognizable (e.g., for level 7, the corresponding portions of carina, both main stem bronchi, esophagus, and pericardium). Secondly, to get a better thoracoscopic view, the lung must be completely collapsed.
Statistical analysis
Numerical data were expressed as mean and standard deviation or median and interquartile range (IQR) according to their distribution. Categorical data were recorded as frequencies and percentages.
Comparison between groups was performed Mann Withney test. Pearson’s correlation index was used to assess relationship between variables. Inter-rater agreement between pN and cN was estimated through the Cohen’s kappa (κ) coefficient. Logistic regression analyses were performed to investigate whether there is a relationship between the risk of having complication and the pre-operative and surgical variables including number of lymph nodes retrieved. Generalized linear model with Gamma-distributed response variables was applied to estimate the relationship between length of stay as dependent variables and possible predictors such as the pre-operative and surgical variables including number of lymph nodes retrieved.
All tests were two-tailed, and a P value <0.05 was considered as statistically significant. Analyses were performed using R version 4.0.1 (The R Project for Statistical Computing).
Results
Demographic, comorbidity, preoperative and postoperative information of the patients is presented in Table 1.
We had no cases of chylothorax and 9 cases of laryngeal recurrent nerve palsy after MLND (6 cases after MLND at station 4L and 3 cases after MLND at station 5).
From May 2012 to December 2020, we operated 525 patients of VATS lobectomy (conversion rate 11.5%) using a multiportal approach: 42 procedures with a 4-port approach, 56 procedures with a 3-port approach and 427 with a 2-port approach.
We analyzed and compared the number of lymph nodes collected during VATS lobectomy, in each station N1 and N2 by side and by type of surgery. The RUL and RML interventions are also considered together to be able to extend the comparison with the data of another author. There was no statistically significant difference in the number of total lymph nodes retrieved either by side or by type of surgical intervention (Figure 1).
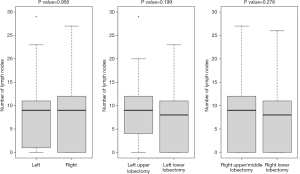
We observed that there is a statistically significant difference in the number of lymph nodes collected at station 3 between patients undergoing RUL + RML vs. RLL and at station 4 between LUL vs. LLL (Table 3).
Table 3
| Station | RUL + RML | RLL | LUL | LLL | P value (RUL + RML vs. RLL) | P value (LUL vs. LLL) |
|---|---|---|---|---|---|---|
| Station 1 | 0 (0–0); (min =0 to max =0) |
0 (0–0); (min =0 to max =0) |
0 (0–0); (min =0 to max =0) |
0 (0–0); (min =0 to max =0) |
0.11 | 0.84 |
| Station 2 | 0 (0–0); (min =0 to max =2) |
0 (0–0); (min =0 to max =2) |
0 (0–0); (min =0 to max =0) |
0 (0–0); (min =0 to max =1) |
0.88 | 0.25 |
| Station 3 | 2 (1–3); (min =0 to max =10) |
1 (0–2); (min =0 to max =10) |
0 (0–0); (min =0 to max =13) |
0 (0–0); (min =0 to max =6) |
<0.01 | 0.92 |
| Station 4 | 0 (0–0); (min =0 to max =5) |
0 (0–0); (min =0 to max =5) |
2 (1–3); (min =0 to max =8) |
1 (0–2); (min =0 to max =6) |
0.27 | <0.01 |
| Station 5 | 0 (0–0); (min =0 to max =1) |
0 (0–0); (min =0 to max =0) |
0 (0–0); (min =0 to max =2) |
0 (0–0); (min =0 to max =2) |
0.45 | 0.65 |
| Station 6 | 2 (1–3); (min =0 to max =9) |
2 (1–3); (min =0 to max =9) |
1 (1–2); (min =0 to max =4) |
1 (1–2); (min =0 to max =6) |
0.27 | 0.54 |
| Station 7 | 0 (0–0); (min =0 to max =4) |
0 (0–1); (min =0 to max =5) |
0 (0–0); min =0 to max =3) |
0 (0–0); (min =0 to max =3) |
<0.01 | <0.01 |
| Station 8 | 0 (0–0); (min =0 to max =6) |
0 (0–1); (min =0 to max =3) |
0 (0–0); (min =0 to max =1) |
1 (0–1.25); (min =0 to max =5) |
<0.01 | <0.01 |
| Station 9 | 3 (1–4); (min =0 to max =23) |
1 (0–2); (min =0 to max =13) |
2 (1–4); (min =0 to max =9) |
1.5 (0–2); (min =0 to max =12) |
<0.01 | <0.01 |
| Station 10 | 1 (0–2); (min =0 to max =11) |
2 (0–3); (min =0 to max =14) |
2 (1–3); (min =0 to max =7) |
2 (1–3); (min =0 to max =8) |
<0.01 | 0.75 |
| Station 11 | 0 (0–1); (min =0 to max =6) |
0 (0–0); (min =0 to max =6) |
0 (0–2); (min =0 to max =7) |
0 (0–1); (min =0 to max =6) |
0.40 | 0.07 |
| Station 12 | 0 (0–0); (min =0 to max =0) |
0 (0–0); (min =0 to max =0) |
0 (0–0); (min =0 to max =0) |
0 (0–0); (min =0 to max =1) |
– | 0.25 |
RUL, righ upper lobectomy; RML, right middle lobectomy; RLL, right lower lobectomy; LUL, left upper lobectomy; LLL, left lower lobectomy.
Lymphadenectomy at stations 7, 8 and 9 is significantly different between RUL + RML vs. RLL and between LUL vs. LLL, respectively. Finally, the difference in ND during RUL + RML vs. RLL is significative also for station 10 (Table 3).
We assessed the accuracy of our lymphadenectomy technique also considering the upstaging rate. Evaluating the value of the Cohen Kappa index (0.292) there is a poor agreement between pN and cN. We recorded 79 cases (15%) in which pN and cN are discordant. We have an upstaging rate: 2.5% and a downstaging rate: 12.6%. In each case of downstaging the involved lymph nodes were N2 (stations 4R and 7).
We found that the only variable associated with a risk of postoperative complications is vital capacity (VC) (OR =0.979; 95% CI: 0.964 to 0.993; P value =0.005). The variables that are instead related with the length of hospitalization are FEV1 (βFEV1=−0.0032; 95% CI: −0.0050 to −0.0013; P value <0.001), VC (βVC =−0.0026; 95% CI: −0.0045 to −0.0006; P value =0.010) and gender-male (βMale =0.0800; 95% CI: 0.0010 to 0.1581; P value =0.046).
The number of lymph nodes removed is therefore not related either to having post-operative complications or to the length of hospitalization.
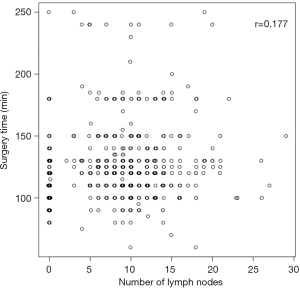
The same can be said about the impact that the number of lymph nodes removed has on the surgical time. There is only a weak relationship (r=0.177) between the number of lymph nodes removed and the surgical time (Figure 2).
Discussion
To better understand the importance of MLND, we believe it is necessary to report a brief historical background.
Regional lymph node dissection it considered as one of the most important part of the surgical treatment of many solid tumors. It was applied first in 1894 by Halsted (35) to surgical treatment of breast cancer during radical mastectomy. Then, it was later applied to the other malignancies such as head and neck cancers, gastrointestinal neoplasms, and melanomas.
Cahan was the first surgeon (36) to introduce, in 1951, the term “radical pneumonectomy” referring to a pneumonectomy with hilar and MLND. In 1960 (37), the same author, published 48 cases of “radical lobectomy” (lobectomy with regional lymph node dissection). His work indicated a procedure that rapidly became the standard surgical treatment for lung cancer.
Nael Martini, reporting his experience on lymph node dissection at Memorial Sloan-Kettering Cancer Center, describe in 1995 (31) the radical MLND previously hypothesized by Cahan as the removal, on the right side, of all paratracheal nodes, all subcarinal nodes and all nodes near the inferior pulmonary ligament. On the left side, node dissection means all nodes in the aorticopulmonary window, subcarinal region and near the inferior pulmonary ligament.
In 1996 the terms “radical” and “mediastinal” were discarded by the International Association for the Study of Lung Cancer (IASLC) to avoid the discount of the importance of evaluating N1 nodes and to avoid erroneous therapeutic benefit meaning from this evaluation (38).
ESTS guidelines define different type of “Radical Mediastinal Lymphadenectomy” according with the extent of dissection.
Systematic nodal dissection (SND) is defined as the dissection and removal of all mediastinal tissue containing the lymph nodes within anatomical landmarks.
The term “sampling” describes instead a lesser excision of fewer nodal stations that seem to be suspect for metastases in presurgical evaluations or intraoperative findings. The term “systemic sampling” is used, otherwise, for a routine biopsy of lymph node stations specified by the surgeon (39).
Finally, it has been proposed also a more selective lymph node dissection. Advanced analysis of the lymph node pathway of metastases had led, in fact, to perform even lobe- or segment-specific nodal dissection or sampling (40).
ESTS’s guidelines determining the extent of node dissection and standardizing MLND according to surgical procedure and NSCLC staging, have established that systematic MLND is mandatory during every pulmonary surgical intervention with oncological radical intent. Lobe-specific systematic node dissection is acceptable for peripheral T1 tumors if hilar and intralobar nodes are negative on frozen sections. It means that at least three hilar and intralobar nodes and three mediastinal nodes should be sampled (41).
It is commonly a matter of discussion the assumption that MLND during open procedure is more accurate than that performed during VATS ones. It is demonstrated (42), regardless of how accuracy is assessed, at least the non-inferiority of VATS MLND to that through an open thoracotomy.
There are many authors who have proved the non-inferiority of MLND via VATS to that through an open thoracotomy considering the amount of lymph nodes retrieved.
D’Amico et al. (43) compared the accuracy and efficacy of MLND during lobectomy via VATS versus thoracotomy. He found that the number of LNs retrieved was similar between the two groups. The same route was for Scott et al. (2), Watanabe et al. (42) and Palade et al. (44). Finally, Medbery et al. (45) and Wang et al. (46) found that MLND via VATS approach is even superior to that via thoracotomy.
If we consider instead the nodal upstaging as index of accuracy, we must consider the study of Boffa et al. (47) who demonstrated that MLND by VATS and thoracotomy has the same upstaging rate (considering only N2 stations).
It remains premature to declare the superiority of a specific type of VATS approach (e.g., uniportal versus biportal versus three or four ports) in lung cancer surgery and in particular for MLND (48). A higher level of evidence is needed, especially in investigating objective benefits and treatment efficacy of the different VATS approaches. In 2018 we reviewed a series of 400 consecutive cases of VATS lobectomy, performed from May 2012 to December 2017, using progressively less ports (4-3-2). To avoid the limit of a small sample size (single center study), the non-randomized and retrospective design of the study, we used a Propensity score analysis to overcome several biases. We can affirm, confirming and reproducing the results of all the series published even in the case of the largest ones such as that of Medbery (45) where 4,437 patients were operated of VATS lobectomy and MLND (Table 4), that there were no significant differences in terms of number of complications, operative time and number of lymph node retrieved between the three groups. Our study did not compare these results with a group of lobectomies performed via thoracotomy (4).
Table 4
| Author | Published year | No. patients | No. ports | Side | No. lymph node (mean value) |
|---|---|---|---|---|---|
| Watanabe (42) | 2005 | 191 | 2 | Right; Left | 32.3*; 28.9* |
| Whitson (49) | 2007 | 59 | 3/4 | Bilateral | 6.3 |
| Denlinger (50) | 2010 | 79 | NA | Bilateral | 7.1 |
| Scott (2) | 2010 | 66 | NA | Bilateral | 15 |
| Merritt (51) | 2013 | 60 | 2/3 | Bilateral | 9.9 |
| Wang (46) | 2014 | 2,703 | NA | Bilateral | 18.03 |
| Palade (44) | 2013 | 32 | 3 | Right; Left | 24; 25.1 |
| Medbery (45) | 2016 | 4,437 | NA | Bilateral | 10.3 |
| Andriolo (4) | 2018 | 400 | 2; 3; 4 | Bilateral | 11; 12.2; 9 |
*, value between upper/middle and lower lobectomy. VATS, video-assisted thoracoscopic surgery.
Another matter of discussion about MLND are the hypothetical benefits of SND over sampling. Due the fact that they are not clear, we believe it is important to adapt on a case-by-case basis, the extent of lymphadenectomy.
There are no RCTs (52,53), comparing one lymph node sampling group to another lymph node dissection group due to different reasons: from obvious ethical considerations to the hazard of statistical biases (54). The few studies published on this subject, enrolling a low number of patients, sometimes concluded giving an important role only to the stage migration of the (expected) survival benefit registered of the SND over the sampling.
Many surgeons, included us at the beginning of our experience, are moved by the common sense that SND lead to an almost absolute nodal staging. But the potential risk of such a dissection can led to a worst postoperative outcome than nodal downstaging itself. The dehiscence of bronchial stump favored by the extensive devitalization of bronchial tree, the chylothorax and bleeding caused by the extensive field of dissection and the increased risk of ARDS or pulmonary edema after resection of lymphatic pathway should led to reflection before performing SND independently by the clinical stage. However, it also true that the preoperative evaluation with CT and PET is not reliable enough due to the always possible presence of micrometastases (55). In fact, Nomori et al. (56) has identified a threshold for detection of lymph node metastases by PET. He found that a tumor focus measuring less than 4 mm in diameter were not recognized by PET. Furthermore, a positive PET needs whenever a pathological evaluation due to its high false positive rate (57). Thus, according with the literature (58), we decided subsequently to perform sampling for the stage I NSCLC and SND for higher ones. We performed 468 VATS lobectomy for stage I (IA + IB) NSCLC. In these patients we performed only mediastinal lymph node sampling.
Swapping a double ND technique: sampling and systematic nodal dissection we tried to evaluate the accuracy of our lymphadenectomy considering first the number of lymph nodes collected. We cannot be sure that each lymph node considered as whole is, in reality, a fragment of that because we use a hybrid technique between grasping and non-grasping technique. However, the fact that our lymphadenectomy series is compatible with that of the most authoritative literature (Table 4) makes us confident that in most cases the lymph nodes considered are whole.
Furthermore, in several cases (peripheral tumor smaller than 2 cm with negative PET) we performed even a lobe-specific lymphadenectomy. This last aspect, considering the technical peculiarities of each type of lobectomy with its own lymphadenectomy, would explain the significant differences in the number of lymph nodes removed considering the same station but in different operations on the same side. Station 3 is more frequently explored during upper and middle lobectomy rather than lower right lobectomy as station 3 receives lymph predominantly from the upper half of the lung (59,60). There is a significative difference in lymphadenectomy in station 4 between LUL versus LLL. The same can be said for lymphadenectomy in stations 7, 8 and 9 between RUL + RML versus RLL and between LUL versus LLL (Table 3). Finally, station 7 is more easily explored after upper left lobectomy, exerting traction on the bronchial stump (32), than after lower left lobectomy and this would explain the significant difference in the number of lymph nodes removed.
There was no statistically significant difference in the number of total lymph nodes retrieved either by side or by type of surgical intervention (Figure 1).
We assessed the accuracy of our lymphadenectomy technique also considering the upstaging rate. Graham et al. (61) showed that the presence of “unexpected” pN2 exists independently of the histology, size, and location of the primary tumor. In fact, up to 20% of cases of T1 <2 cm has an incidence of pN2 >20%. This is even more evident when we consider the upstaging rate (cN0 to pN1–N2 or cN1 to pN2) after lung cancer surgery. It is known, in fact, that up to 38% of patients with pN2 also do not have consensual pN1 due to skip metastases.
The nodal upstaging after surgical resection provides important information about prognosis and may influence the use of adjuvant treatment and, consequently, survival (62). Detection of unsuspected lymph node metastases depends on the extent of lymph node removal: dissection or sampling. However, MLND is not performed routinely in all centers. In a large study using the Nationwide Inpatient Sample (NIS) database, the overall lymphadenectomy rate was 56% in 222,233 patients (63). Thus, the data of nodal upstaging are not ever comparable between different centers.
Evaluating the value of the Cohen Kappa index (0.292) there is a poor agreement between pN and cN. We recorded 79 cases (15%) in which pN and cN are discordant. We have an upstaging rate: 2.5% and a downstaging rate: 12.6%.
In each case of downstaging the involved lymph nodes were N2 (stations 4R and 7). This may be because we have recently started mediastinal staging with EBUS/TBNA, and we still consider those results as cN. We consider our mediastinal staging with EBUS/TBNA as cN due to the fact we are at the beginning of learning curve. Thus, it could be considered, in our opinion, as a staging with PET scan with consequently high NPV. Instead, TBNA, EBUS-FNA and EUS-FNA, if performed by experienced surgeons have high specificity and low NPV.
We also investigate for potential predictors of postoperative complications. We found that the number of lymph nodes removed is not related either to having post-operative complications or to the length of hospitalization.
The same can be said about the impact that the number of lymph nodes removed has on the surgical time. There is only a weak relationship (r=0.177) between the number of lymph nodes removed and the surgical time (Figure 2).
We have not had any case of chylothorax due to MLND (both sampling and systematic). Developing a VATS technique after a long-lasting training and learning tips and tricks of the procedure permits us to avoid this difficult-to-treat complication. Conversely, during our surgical activity we registered 9 cases of laryngeal recurrent palsy after left procedure (6 cases after MLND at station 4L and 3 cases after MLND at station 7). To prevent that complication, we learned to use properly the energy-based devices and to better expose the recurrent nerve and its branches. To avoid putting the uncovered tip in contact with the nerve we prefer Ligasure® over Harmonic® and Sonicision® when performing MLND at station 5 and 6.
Otherwise, when performing MLND at station 4L we progressively adopted the technique described by Nagashima et al. Using a silk tread to traction the recurrent laryngeal nerve the nodal dissection could be safer and more effective thanks to a better view.
Finally, we reckon that analysis describing short-term and (especially) long-term survival are beyond the purpose of our manuscript. Due to the retrospective study design and the monocentric experience reported we are not able to do an accurate estimate of the impact that different types of MLND have on the survival rate after VATS lobectomy for NSCLC.
Conclusions
According with our anatomical-surgical review and data reported we can affirm that VATS approach could permit us to be accurate and effective in treating lung cancer patients. We reckon that we should learn further to become more skilled at performing an adequate lymph node dissection.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Chiappetta and Francesco Facciolo) for the series “Lymphadenectomy during VATS and RATS: state of the art” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://vats.amegroups.com/article/view/10.21037/vats-21-27/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-21-27/coif). The series “Lymphadenectomy during VATS and RATS: state of the art” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roviaro G, Varoli F, Rebuffat C, et al. Major pulmonary resections: pneumonectomies and lobectomies. Ann Thorac Surg 1993;56:779-83. [Crossref] [PubMed]
- Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010;139:976-81; discussion 981-3. [Crossref] [PubMed]
- Andriolo LG, Lopez C, Di Rienzo G. VATS Biportal Left Pneumonectomy. 2019; [Crossref]
- Andriolo LG, Lopez C, Gregori D, et al. Pros-cons debate about the role and evolution of bi-portal video-assisted thoracoscopic surgery. Shanghai Chest 2018;2:67. [Crossref]
- Rami-Porta R, Wittekind C, Goldstraw P, et al. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25-33. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-e313S.
- D'Cunha J, Herndon JE 2nd, Herzan DL, et al. Poor correspondence between clinical and pathologic staging in stage 1 non-small cell lung cancer: results from CALGB 9761, a prospective trial. Lung Cancer 2005;48:241-6. [Crossref] [PubMed]
- Ziyade S, Pinarbasili NB, Ziyade N, et al. Determination of standard number, size and weight of mediastinal lymph nodes in postmortem examinations: reflection on lung cancer surgery. J Cardiothorac Surg 2013;8:94. [Crossref] [PubMed]
- Reichert M, Steiner D, Kerber S, et al. A standardized technique of systematic mediastinal lymph node dissection by video-assisted thoracoscopic surgery (VATS) leads to a high rate of nodal upstaging in early-stage non-small cell lung cancer. Surg Endosc 2016;30:1119-25. [Crossref] [PubMed]
- Wilson JL, Louie BE, Cerfolio RJ, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg 2014;97:1901-6; discussion 1906-7. [Crossref] [PubMed]
- Irons JF, Martinez G. Anaesthetic considerations for non-intubated thoracic surgery. J Vis Surg 2016;2:61. [Crossref] [PubMed]
- Carrott PW Jr, Jones DR. Teaching video-assisted thoracic surgery (VATS) lobectomy. J Thorac Dis 2013;5:S207-11. [PubMed]
- Hansen HJ, Petersen RH. Video-assisted thoracoscopic lobectomy using a standardized three-port anterior approach - The Copenhagen experience. Ann Cardiothorac Surg 2012;1:70-6. [PubMed]
- Decaluwe H, Sokolow Y, Deryck F, et al. Thoracoscopic tunnel technique for anatomical lung resections: a 'fissure first, hilum last' approach with staplers in the fissureless patient. Interact Cardiovasc Thorac Surg 2015;21:2-7. [Crossref] [PubMed]
- De Giacomo T, Venuta F, Rendina EA. Role of lymphadenectomy in the treatment of clinical stage I non-small cell lung cancer. Thorac Surg Clin 2007;17:217-21. [Crossref] [PubMed]
- De Leyn P, Lardinois D, Van Schil P, et al. European trends in preoperative and intraoperative nodal staging: ESTS guidelines. J Thorac Oncol 2007;2:357-61. [Crossref] [PubMed]
- Amer K, Khan AZ, Singh N, et al. Video-assisted thoracic surgery systematic mediastinal nodal dissection and stage migration: impact on clinical pathway. Eur J Cardiothorac Surg 2011;40:1474-81. [Crossref] [PubMed]
- Kim HK, Choi YS, Kim J, et al. Outcomes of unexpected pathologic N1 and N2 disease after video-assisted thoracic surgery lobectomy for clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2010;140:1288-93. [Crossref] [PubMed]
- Cosgun T, Kaba E, Ayalp K, et al. Factors on nodal up-staging in clinical N0 adenocarcinoma patients who had minimally invasive anatomic lung resections. Mini-invasive Surg 2019;3:32. [Crossref]
- Nohl HC. An investigation into the lymphatic and vascular spread of carcinoma of the bronchus. Thorax 1956;11:172-85. [Crossref] [PubMed]
- Naruke T, Suemasu K, Ishikawa S. Lymph node mapping and curability at various levels of metastasis in resected lung cancer. J Thorac Cardiovasc Surg 1978;76:832-9. [Crossref] [PubMed]
- Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997;111:1718-23. [Crossref] [PubMed]
- Watanabe S, Ladas G, Goldstraw P. Inter-observer variability in systematic nodal dissection: comparison of European and Japanese nodal designation. Ann Thorac Surg 2002;73:245-8; discussion 248-9. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Liu C, Pu Q, Guo C, et al. Non-grasping en bloc mediastinal lymph node dissection for video-assisted thoracoscopic lung cancer surgery. BMC Surg 2015;15:38. [Crossref] [PubMed]
- Guo C, Xia L, Mei J, et al. A propensity score matching study of non-grasping en bloc mediastinal lymph node dissection versus traditional grasping mediastinal lymph node dissection for non-small cell lung cancer by video-assisted thoracic surgery. Transl Lung Cancer Res 2019;8:176-86. [Crossref] [PubMed]
- Di Rienzo G, Surrente C, Lopez C, et al. Tips and tricks in video-assisted thoracoscopic surgery lobectomy. Future Oncol 2016;12:35-8. [Crossref] [PubMed]
- Isaka M, Kondo H, Maniwa T, et al. Boundary between N1 and N2 Lymph Node Descriptors in the Subcarinal Zone in Lower Lobe Lung Cancer: A Brief Report. J Thorac Oncol 2016;11:1176-80. [Crossref] [PubMed]
- Watanabe A, Miyajima M, Mishina T, et al. Video-assisted thoracoscopic surgery node dissection for lung cancer treatment. Surg Today 2017;47:1419-28. [Crossref] [PubMed]
- Martini N. Mediastinal Lymph Node Dissection for Lung Cancer. Penfield Faber L. Chest Surgery Clinics of North America 1995;5:189-203. [PubMed]
- Nagashima T. Thoracoscopic left mediastinal lymph node dissection. Ann Transl Med 2016;4:10. [PubMed]
- Baste JM, Haddad L, Melki J, et al. Anterior subcarinal node dissection on the left side using video thoracoscopy: an easier technique. Ann Thorac Surg 2015;99:e99-e101. [Crossref] [PubMed]
- Decaluwe H, Petersen RH, Hansen H, et al. Major intraoperative complications during video-assisted thoracoscopic anatomical lung resections: an intention-to-treat analysis. Eur J Cardiothorac Surg 2015;48:588-98; discussion 599. [Crossref] [PubMed]
- Halsted WS. I. The Results of Operations for the Cure of Cancer of the Breast Performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann Surg 1894;20:497-555. [Crossref] [PubMed]
- Cahan WG, Watson WL, Pool JL. Radical pneumonectomy. J Thorac Surg 1951;22:449-73. [Crossref] [PubMed]
- Cahan WG. Radical lobectomy. J Thorac Cardiovasc Surg 1960;39:555-72. [Crossref] [PubMed]
- Goldstraw P. Report on the international workshop on intrathoracic staging, London, October 1996. Lung Cancer 1997;18:107-11. [Crossref]
- Keller SM, Adak S, Wagner H, et al. Mediastinal lymph node dissection improves survival in patients with stages II and IIIa non-small cell lung cancer. Eastern Cooperative Oncology Group. Ann Thorac Surg 2000;70:358-65; discussion 365-6. [Crossref] [PubMed]
- Asamura H, Nakayama H, Kondo H, et al. Lobe-specific extent of systematic lymph node dissection for non-small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J Thorac Cardiovasc Surg 1999;117:1102-11. [Crossref] [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Watanabe A, Koyanagi T, Ohsawa H, et al. Systematic node dissection by VATS is not inferior to that through an open thoracotomy: a comparative clinicopathologic retrospective study. Surgery 2005;138:510-7. [Crossref] [PubMed]
- D'Amico TA, Niland J, Mamet R, et al. Efficacy of mediastinal lymph node dissection during lobectomy for lung cancer by thoracoscopy and thoracotomy. Ann Thorac Surg 2011;92:226-31; discussion 231-2. [Crossref] [PubMed]
- Palade E, Passlick B, Osei-Agyemang T, et al. Video-assisted vs open mediastinal lymphadenectomy for Stage I non-small-cell lung cancer: results of a prospective randomized trial. Eur J Cardiothorac Surg 2013;44:244-9; discussion 249. [Crossref] [PubMed]
- Medbery RL, Gillespie TW, Liu Y, et al. Nodal Upstaging Is More Common with Thoracotomy than with VATS During Lobectomy for Early-Stage Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2016;11:222-33. [Crossref] [PubMed]
- Wang W, Yin W, Shao W, et al. Comparative study of systematic thoracoscopic lymphadenectomy and conventional thoracotomy in resectable non-small cell lung cancer. J Thorac Dis 2014;6:45-51. [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-53; discussion 353. [Crossref] [PubMed]
- Sihoe ADL. Uniportal Lung Cancer Surgery: State of the Evidence. Ann Thorac Surg 2019;107:962-72. [Crossref] [PubMed]
- Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;83:1965-70. [Crossref] [PubMed]
- Denlinger CE, Fernandez F, Meyers BF, et al. Lymph node evaluation in video-assisted thoracoscopic lobectomy versus lobectomy by thoracotomy. Ann Thorac Surg 2010;89:1730-5; discussion 1736. [Crossref] [PubMed]
- Merritt RE, Hoang CD, Shrager JB. Lymph node evaluation achieved by open lobectomy compared with thoracoscopic lobectomy for N0 lung cancer. Ann Thorac Surg 2013;96:1171-7. [Crossref] [PubMed]
- Izbicki JR, Passlick B, Pantel K, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: results of a prospective randomized trial. Ann Surg 1998;227:138-44. [Crossref] [PubMed]
- Fibla JJ, Cassivi SD, Decker PA, et al. Validation of the lung cancer staging system revisions using a large prospective clinical trial database (ACOSOG Z0030). Eur J Cardiothorac Surg 2013;43:911-4. [Crossref] [PubMed]
- Massard G, Ducrocq X, Kochetkova EA, et al. Sampling or node dissection for intraoperative staging of lung cancer: a multicentric cross-sectional study. Eur J Cardiothorac Surg 2006;30:164-7. [Crossref] [PubMed]
- Oda M, Watanabe Y, Shimizu J, et al. Extent of mediastinal node metastasis in clinical stage I non-small-cell lung cancer: the role of systematic nodal dissection. Lung Cancer 1998;22:23-30. [Crossref] [PubMed]
- Nomori H, Watanabe K, Ohtsuka T, et al. The size of metastatic foci and lymph nodes yielding false-negative and false-positive lymph node staging with positron emission tomography in patients with lung cancer. J Thorac Cardiovasc Surg 2004;127:1087-92. [Crossref] [PubMed]
- Cerfolio RJ, Ojha B, Bryant AS, et al. The role of FDG-PET scan in staging patients with nonsmall cell carcinoma. Ann Thorac Surg 2003;76:861-6. [Crossref] [PubMed]
- Mitsos S, Panagiotopoulos N, Patrini D, et al. Is systematic lymph node dissection mandatory or is sampling adequate in patients with stage I non-small-cell lung cancer? Interact Cardiovasc Thorac Surg 2019;28:550-4. [Crossref] [PubMed]
- Okada Y, Ito M, Nagaishi Ch. Anatomical study of the pulmonary lymphatics. Lymphology 1979;12:118-24. [PubMed]
- Riquet M, Hidden G, Debesse B. Direct lymphatic drainage of lung segments to the mediastinal nodes. An anatomic study on 260 adults. J Thorac Cardiovasc Surg 1989;97:623-32. [Crossref] [PubMed]
- Graham AN, Chan KJ, Pastorino U, et al. Systematic nodal dissection in the intrathoracic staging of patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 1999;117:246-51. [Crossref] [PubMed]
- Butts CA, Ding K, Seymour L, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol 2010;28:29-34. [Crossref] [PubMed]
- Ellis MC, Diggs BS, Vetto JT, et al. Intraoperative oncologic staging and outcomes for lung cancer resection vary by surgeon specialty. Ann Thorac Surg 2011;92:1958-63; discussion 1963-4. [Crossref] [PubMed]
Cite this article as: Andriolo LG, Alunni Fegatelli D, Spagnoli A, Di Rienzo G. VATS mediastinal lymph node dissection: surgical technique and literature review. Video-assist Thorac Surg 2022;7:5.


