NiVATS sympathectomy for hyperhidrosis: should I stay or should I go? A Narrative Review
Introduction
Non-intubated video-assisted thoracoscopic surgery (NiVATS) was first reported by Jacobaeus in 1922 (1). During the last 16 years, this procedure has become more popular and has been introduced in several centers worldwide (2-4). It has proved to be a feasible and safe technique for different thoracic surgery operations (5). In a randomized study, Liu et al. compared NiVATS procedures under epidural anesthesia with thoracic surgery operations under general anesthesia (VATS). For bullae resection, wedge resection and lobectomy, they showed a shorter postoperative fasting time, a shorter duration of antibiotic use and a shorter length of hospital stay (6). In addition, NiVATS combined with intravenous analgosedation, local infiltration anesthesia and intercostal nerve blocks was proven feasible as well (7) and to provide further benefits in reducing postoperative discomforts such as vomiting and nausea, sore throat and pain and reduces intubation and ventilation related lung injuries (8). An intrathoracic vagal block by infiltration near the vagal nerve inhibits the cough reflex during thoracoscopic manipulation of the lung (7).
The advantages of NiVATS such as lower risks, lower costs and outpatient management leads to more acceptance of a surgical treatment for non-life risk diseases like hyperhidrosis. There are several studies demonstrated the feasibility, safety and effectiveness of NiVATS sympathectomy for hyperhidrosis (9-15). However, surgeons and anesthesiologists face new challenges during NiVATS, such as coughing, any movements by the awake patient, mediastinal shift and diaphragmatic displacement. This review aims to summarize the current literature about NiVATS for patients with hyperhidrosis. We present the following article in accordance with the Narrative Review reporting checklist (available at https://dx.doi.org/10.21037/vats-21-11).
Methods
We performed a literature search on PubMed with the terms ‘nonintubated + sympathectomy + hyperhidrosis’ and ‘awake + sympathectomy + hyperhidrosis’. Only original articles were included. Case series with less than 10 patients were excluded. One article was excluded because it was only available in Spanish (16). Therefore, seven articles were included in our review (Table 1).
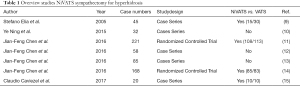
Full table
NiVATS for sympathectomy
Patient selection
In all studies, healthy patients with primary hyperhidrosis were included. Caviezel et al. performed one NiVATS in a patient with facial blushing (15).
Anesthesia
Elia et al. performed the NiVATS procedure without any sedation or intravenous analgetic medication (9). In all other studies dexmedetomidine or propofol in combination with sufentanil, remifentanyl or fentanyl was administered intravenously with boluses or by target-controlled infusion, as required for patient comfort.
Oxygen was administered continuously through a standard nasal cannula or a face mask with a rate of 2–5 L/min. Chen et al. performed one study using a laryngeal mask in some cases (12).
Surgery and local anesthesia
Patients were placed in a semi-prone position for each side/procedure and mild anti-Trendelenburg inclination (9) or in a prone position with both arms abducted (15).
Elia et al. performed a two port thoracoscopy while the other authors all describe an uniportal thoracoscopy.
For local anesthesia, when described, mepivacaine, lidocaine and/or ropivacaine were used for the skin and intercostal space. Before cutting the sympathic chain, lidocaine was applied to both sides in the subpleural space through an endoscopic syringe. The cutting of the sympathic chain was performed using scissors or electrocautery hook. Additionally, Caviezel et al. performed a vagal block with lidocaine to inhibit the cough reflex during thoracoscopy.
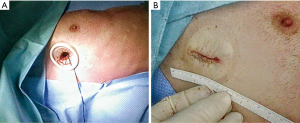
The number and level of insertion of the 5 mm ports varied between centers. Caviezel et al. used a wound protector (Figure 1). Therefore, the entry point was chosen between the 3th and 4th intercostal space in the mid-axillary line or anterior of it. In contrast, Chen et al. performed a transareolar access.
For re-expansion of the lung, most authors used a temporary chest tube, which was connected to a suction device under thoracoscopic visualization. Some authors describe manually ventilation with continuous positive pressure by the anesthesiologist to prevent a pneumothorax—provided a laryngeal mask in situ. Once the procedure was completed, the chest tubes were removed. After a surveillance of 1 to 2 hours, the patients could be transferred to the ward. Most of them were dismissed from the hospital on the same day or on the first postoperative day, once the postoperative chest X-ray showed no relevant pneumothorax.
Data collections
The collected data in all studies were as follows; ‘In operating room time’, recovery time, palmar temperature rise, resolution of palmar hyperhidrosis, complications after surgery (pneumothorax, compensatory sweating, Horner syndrome, recurrence, bleeding), hospital stay and costs.
Results
The overall conclusion of the studies was that, that NiVATS is equal safe as VATS. The findings showed that there were no disadvantages compared to VATS. The patients who underwent NiVATS, suffered no residual pain and experienced a faster recovery after surgery (Table 2).

Full table
There was no mortality or the need to convert to an open procedure in both groups. The studies showed an equal operating time but a shorter in-operating-room time, a shorter length of stay and lower costs for NiVATS (especially in relation to anesthesia and hospitalization) (Table 3).
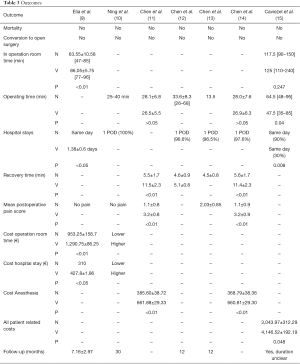
Full table
After thoracoscopic sympathectomy, patients had an increased quality of life (QOL), regardless of the surgical technique or type of anesthesia. In most articles, the follow up of patients was between operation and up to 12-month postoperatively. However, the main differentiation was that satisfaction of the NiVATS-group was significantly higher 24 hours postoperatively (9). Regarding the long-term follow-up, there was no difference in quality of life, resolution of symptoms or compensatory sweating. No patient showed a recurrence of symptoms.
Discussion
VATS sympathectomy is a worldwide accepted and evidence-based treatment for primary hyperhidrosis (17). The thoracoscopy is usually performed as uni- or biportal, depending on surgeons’ preference. Mostly, resection or diversion is limited to the levels T3 or T4.
In a recent meta-analysis, comparing NiVATS with VATS in 1,684 cases, Zhang et al. described a significantly lower complication rate in NiVATS (5). Studies comparing NiVATS and VATS in sympathectomy showed no differences regarding efficiency and operative morbidity (9,11,14,15).
In terms of anesthesia and intubation time, ventilation associated complications (sore throat, nausea, vomiting), postoperative complications (pneumonia, air leak, pain), hospital stay and perioperative mortality rate, NiVATS with regional or local anesthesia has been shown to be equally safe compared with VATS procedures. In addition, there are usually lower costs due to shorter length of hospital stay, lesser equipment required such as double lumen intubation tube etc. (5,9-15). The more rapid patient recovery after NiVATS may also allow an outpatient management. However, this seems to be relevant in case of sympathectomy (15), but can be questioned in cases, where a postoperative chest tube might outlast the immediate faster recovery from anesthesia. Nevertheless, surgeons and anesthesiologists might have an increased level of stress while operating on an awake or at least non-intubated patient.
In addition, some authors showed significant less inflammatory cytokines (tumor necrosis factor alpha and C-reactive protein) (6), lymphocyte activity (18) and reduced endocrine response (19) after NiVATS as after VATS. This might explain the fewer postoperative respiratory complications, shorter postoperative fasting time, shorter duration of antibiotic use and shorter hospital stay generally in NiVATS patients.
Another issue to consider is that before performing NiVATS, it is essential to raise the patient’s awareness of possible intraoperative discomfort. They should understand that they may experience some shortness of breath, cough reflex, pain and the surgeon’s manipulations. They need to be able to cooperate well. Therefore, the patient has to be carefully selected, as shown in Table 4.
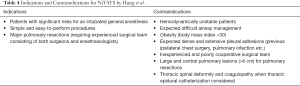
Full table
Indications and Contraindications for NiVATS as described by Hung et al. (20). The words highlighted in bold are well applicable to sympathectomy (Table 4).
Thoracoscopic sympathectomy is a simple and easy to perform procedure, although there are different techniques (number of ports, access, extent of resection, technique of resection). It is therefore appropriate to start a NiVATS program, providing a fast learning curve regarding the technique (15). Provided, that the team has extensive experience in VATS, surgeons and anesthesiologists can focus on local anaesthesia and the intrathoracic situation under spontaneous ventilation.
Most of the reviewed studies are retrospective and performed in different high volume centers. Current investigations of large databases and multinational studies comparing NiVATS and VATS for different indications show further promising results (21).
Caviezel et al. showed that the learning curve in a well prepared team is fast without any complications compared to VATS.
Especially in times of increasingly emerging ERAS programs in thoracic surgery (22), NiVATS might help to manage thoracoscopic sympathectomy as an outpatient procedure. Additionally, thoracoscopic sympathectomy might be an ideal immersion for NiVATS.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Francesco Guerrera, Paolo Albino Ferrari and Roberto Crisci) for the series “Non-Intubated Thoracic Surgery. A Global Perspective” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review Reporting Checklist. Available at https://dx.doi.org/10.21037/vats-21-11
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/vats-21-11). The series “Non-Intubated Thoracic Surgery. A Global Perspective” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jacobaeus HC. The practical importance of thoracoscopy in surgery of the chest. Surg Gynecol Obstet 1922;289-96.
- Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761-8. [Crossref] [PubMed]
- Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg 2011;254:1038-43. [Crossref] [PubMed]
- Guo Z, Shao W, Yin W, et al. Analysis of feasibility and safety of complete video-assisted thoracoscopic resection of anatomic pulmonary segments under non-intubated anesthesia. J Thorac Dis 2014;6:37-44. [PubMed]
- Zhang K, Chen HG, Wu WB, et al. Non-intubated video-assisted thoracoscopic surgery vs. intubated video-assisted thoracoscopic surgery for thoracic disease: a systematic review and meta-analysis of 1,684 cases. J Thorac Dis 2019;11:3556-68. [Crossref] [PubMed]
- Liu J, Cui F, Li S, et al. Nonintubated video-assisted thoracoscopic surgery under epidural anesthesia compared with conventional anesthetic option: a randomized control study. Surg Innov 2015;22:123-30. [Crossref] [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic surgery using regional anesthesia and vagal block and targeted sedation. J Thorac Dis 2014;6:31-6. [PubMed]
- Gonzalez-Rivas D, Bonome C, Fieira E, et al. Non-intubated video-assisted thoracoscopic lung resections: the future of thoracic surgery? Eur J Cardiothorac Surg 2016;49:721-31. [Crossref] [PubMed]
- Elia S, Guggino G, Mineo D, et al. Awake one stage bilateral thoracoscopic sympathectomy for palmar hyperhidrosis: a safe outpatient procedure. Eur J Cardiothorac Surg 2005;28:312-7; discussion 317. [Crossref] [PubMed]
- Ning Y, Wang Y, Tao X, et al. Single-Port Endoscopic Thoracic Sympathectomy with Monitored Anesthesia Care: A More Promising Procedure for Palmar Hyperhidrosis. World J Surg 2015;39:2269-73. [Crossref] [PubMed]
- Chen JF, Lin M, Chen P, et al. Nonintubated Needlescopic Thoracic Sympathectomy for Primary Palmar Hyperhidrosis: A Randomized Controlled Trial. Surg Laparosc Endosc Percutan Tech 2016;26:328-33. [Crossref] [PubMed]
- Chen J, Lin J, Tu Y, et al. Nonintubated Transareolar Endoscopic Thoracic Sympathectomy with a Flexible Endoscope: Experience of 58 Cases. Ann Thorac Cardiovasc Surg 2016;22:12-9. [Crossref] [PubMed]
- Chen JF, Lin JB, Tu YR, et al. Nonintubated transareolar single-port thoracic sympathicotomy with a needle scope in a series of 85 male patients. Surg Endosc 2016;30:3447-53. [Crossref] [PubMed]
- Chen J, Du Q, Lin M, et al. Transareolar Single-Port Needlescopic Thoracic Sympathectomy Under Intravenous Anesthesia Without Intubation: A Randomized Controlled Trial. J Laparoendosc Adv Surg Tech A 2016;26:958-64. [Crossref] [PubMed]
- Caviezel C, Schuepbach R, Grande B, et al. Establishing a non-intubated thoracoscopic surgery programme for bilateral uniportal sympathectomy. Swiss Med Wkly 2019;149:w20064 [Crossref] [PubMed]
- Mier-Odriozola JM. Sedated non-intubated bilateral thoracoscopic sympathectomy R3-R4. Gac Med Mex 2016;152:228-30. [PubMed]
- Cerfolio RJ, De Campos JR, Bryant AS, et al. The Society of Thoracic Surgeons expert consensus for the surgical treatment of hyperhidrosis. Ann Thorac Surg 2011;91:1642-8. [Crossref] [PubMed]
- Vanni G, Tacconi F, Sellitri F, et al. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg 2010;90:973-8. [Crossref] [PubMed]
- Tacconi F, Pompeo E, Sellitri F, et al. Surgical stress hormones response is reduced after awake videothoracoscopy. Interact Cardiovasc Thorac Surg 2010;10:666-71. [Crossref] [PubMed]
- Hung MH, Hsu HH, Cheng YJ, et al. Nonintubated thoracoscopic surgery: state of the art and future directions. J Thorac Dis 2014;6:2-9. [PubMed]
- Pompeo E, Rogliani P, Atinkaya C, et al. Nonintubated surgical biopsy of undetermined interstitial lung disease: a multicentre outcome analysis. Interact Cardiovasc Thorac Surg 2019;28:744-50. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
Cite this article as: Haessig G, Caviezel C. NiVATS sympathectomy for hyperhidrosis: should I stay or should I go? A Narrative Review. Video-assist Thorac Surg 2021;6:29.