
Narrative review of non-intubated video-assisted thoracic surgery (Ni-VATS) for lung cancer: a focus on lobectomy
Background
Video-assisted thoracic surgery (VATS) lobectomy is now a standard treatment for lung cancer. Nonetheless, modifications to modern VATS, such as awake or non-intubated VATS (Ni-VATS), may allow thoracic surgeons to utilize a less invasive approach for cancer treatment. In 2004, Pompeo and colleagues first proposed that solitary lung nodules could be resected via awake VATS, with the goal of preventing intubation-related complications and ventilator-induced lung injury (1). Further, the successful use of awake VATS in major operations such as lobectomy has opened a new window for the treatment of lung cancer (2). Chen et al. first described the detailed techniques of Ni-VATS lobectomy for lung cancer in 2011 (3). In the past few years, although awake VATS or Ni-VATS has been widely applied in minor thoracic surgeries such as pleural biopsy, wedge resection, lung volume reduction surgery, and bullectomy (4-6), reports concerning Ni-VATS lobectomy are much less common. To summarize current evidence and identify areas that warrant further study, we conducted a narrative review of eligible studies to compare perioperative outcomes and oncological outcomes of Ni-VATS and intubated VATS (IVATS) lobectomy among patients with lung cancer. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/vats-21-20).
Methods
Search strategy and inclusion criteria
We performed a literature search for studies involving Ni-VATS lobectomy for lung cancer across PubMed, Embase, and the Cochrane library databases, from their earliest record to November 2020. The titles and abstracts were reviewed for relevant references. Only literature written in English or available in full was included. The search criteria were as follows: (video-assisted thoracic surgery OR VATS OR thoracoscopy OR thoracoscopic surgery) AND (nonintubated OR non-intubated OR awake) AND lobectomy. Only abstracts specifically addressing Ni-VATS as the main subject were enrolled for subsequent evaluation. Other inclusion criteria were as follows: (I) randomized controlled trials (RCTs) or observational studies comparing Ni-VATS with conventional IVATS in patients undergoing lobectomy for the treatment of lung cancer; (II) studies with sufficient data for extraction of main postoperative outcomes; and (III) the most recent study in a group among duplicate studies. We excluded (I) studies that did not compare Ni-VATS and IVATS; (II) studies in which patients in both groups underwent different surgical procedures; (III) studies in which patients were not diagnosed with lung cancer or did not undergo lobectomy; (IV) letters, editorials, case reports, expert opinions, and reviews; and (V) studies from which relevant data could not be extracted.
Data extraction and assessment of risk of bias
Two authors assessed each retrieved article based on the eligibility criteria. When multiple studies contained overlapping data, the most informative study was included. Data were independently extracted by two authors onto a standardized data extraction sheet. The first authors, publication year, sample size, details of the Ni-VATS and IVATS approaches, and comparative outcomes were systematically recorded. The outcomes were summarized using descriptive statistics such as means and frequencies. Vote counting based on the direction of effect was also used for synthesis. The methodological quality of enrolled studies was evaluated using the Jadad scale for RCTs and the Newcastle-Ottawa Scale (NOS) for observational studies (7). The Jadad scale reflects randomization (2 points), blinding of the studies (2 points), and withdrawals (1 point). Scores range from 0 to 5, and a higher Jadad score indicates better methodological quality. The NOS contains three factors: participant selection, comparability of the study groups, and outcomes. NOS scores of 0–9 (allocated as stars) were assigned to each observational study.
Results
Characteristics of included articles
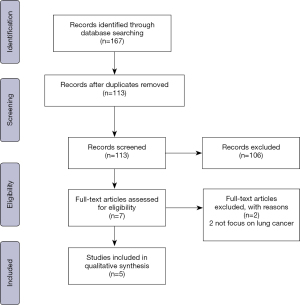
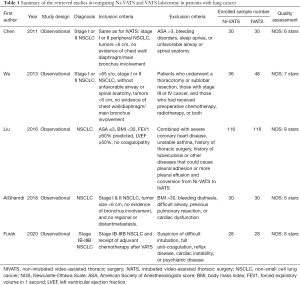
We retrieved 113 unique articles based on a review of their titles and abstracts; from these, seven articles were examined further (Figure 1). We excluded two studies that did not focus on lung cancer. Ultimately, the review included 502 patients from five retrospective cohort studies (Table 1) (3,8-11). Generally, in these studies, patients considered appropriate for Ni-VATS lobectomy met the same criteria required for intubated single-lung ventilation, including clinically early-stage lung cancer, tumors less than 6 cm, no evidence of chest wall/diaphragm/main bronchus involvement, and American Society of Anesthesiologists score ≤3. One study by Wu et al. focused on geriatric patients with lung cancer and only included patients aged 65 years or older (9), while another study by Furák et al. included patients who had received adjuvant chemotherapy after VATS (10). Common exclusion criteria included expected pleural adhesion, unfavorable airway, and conditions inappropriate for thoracic epidural anesthesia (TEA) (e.g., unfavorable spinal anatomy and coagulopathy).
Full table
Anesthetic protocols of included studies
The anesthetic and surgical protocols of Ni-VATS varied among the study groups (Table 2) (3,8-11). In the non-intubated group, three studies applied TEA for regional analgesia and two studies used intercostal nerve blocks (ICNBs). Propofol infusion was used for sedation in all studies, with different adjuvants such as fentanyl, remifentanil, and dexmedetomidine. Four studies used oxygen face masks for breathing support, while two studies applied laryngeal mask airways (LMAs). All studies applied vagal nerve blocks for cough suppression. For the intubated group, four studies utilized double-lumen endotracheal tubes (DLTs) for single-lung ventilation and one study did not mention the type of tube used.

Full table
Perioperative outcomes of included studies
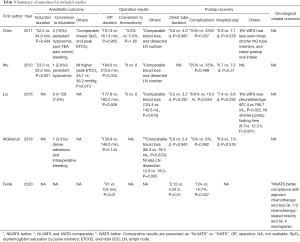
The results of perioperative assessments including anesthetic outcome, operative results, and postoperative recovery are summarized in Table 3. One study by Chenet al. (3) reported comparable induction duration between the intubated and non-intubated groups, while another study (9) involving geriatric patients reported a shorter induction time in the non-intubated group (23.0 vs. 33.2 min;P=0.001). The available intraoperative physiological data were generally comparable between the two groups except for higher PaCO2 without adverse effects noted in non-intubated geriatric patients (45.1 vs. 36.2 mmHg, P=0.013) in Wu et al.’s study (9). The conversion rate to intubation was between 2.8% and 10%, and the reasons for conversion included persistent hypoxemia or hypercapnia, significant mediastinal movement, bleeding, extensive pleural adhesions, and poor TEA pain control.
Full table
The operation duration was comparable in most studies, except for one study reporting shorter surgical duration in the non-intubated group (10). Blood loss was also comparable between the two groups in four studies (3,8,9,11). One study reported less lymph node dissection in the Ni-VATS group (8).
Two studies reported faster postoperative oral intake in non-intubated patients (3,11). Most postoperative recovery results were comparable between the two groups in all studies, except for the study by Furák et al. (10) that reported shorter chest tube duration (2.12 vs. 4.33 d; P<0.01) and fewer postoperative complications (0% vs. 15.7%; P=0.027) in the non-intubated group. In addition, Liu et al. (11) observed less pleural drainage and shorter hospital stays (7.4 vs. 8.5 d; P=0.035) among non-intubated patients. No postoperative mortality was reported in any of the included studies.
Oncological outcomes of Ni-VATS and IVATS
None of the studies compared mid-term or long-term oncological outcomes such as survival or recurrence between the two groups. Only one study by Furák and colleagues compared oncological treatment-related responses between non-intubated and intubated VATS (10). They retrospectively reviewed data for patients with stage IB-IIIB non-small-cell lung carcinoma (NSCLC) who had undergone non-intubated or intubated VATS lobectomy and received adjuvant chemotherapy. Their findings indicated that more non-intubated patients completed the planned chemotherapy protocol than intubated patients (92% vs. 71%, P=0.035). There was also a lower incidence of grade 1/2 toxicity (0% vs. 16%, P=0.03) and grade 4 neutropenia in the non-intubated group. They concluded that the non-intubated approach resulted in improved adjuvant chemotherapy compliance and lower toxicity rates after VATS lobectomy.
Discussion
In this review, we systematically explored current evidence concerning Ni-VATS and IVATS for lobectomy in patients with lung cancer. Five retrospective comparative cohort studies were included. The evidence shows that most perioperative outcomes were comparable between the non-intubated and intubated approaches. Some studies reported faster postoperative recovery in different aspects such as faster oral intake, shorter chest tube drainage duration, and shorter hospital stay in the non-intubated group. Only one study presented oncological treatment-related outcomes, reporting that a non-intubated approach may enhance compliance with adjuvant chemotherapy and decrease chemotherapy-related toxicity.
Patient selection for Ni-VATS lobectomy for lung cancer
There is no consensus regarding inclusion criteria specific for a non-intubated approach to VATS lobectomy. Although VATS lobectomy without tracheal intubation was first reported by Al-Abdullatief et al. in 2007 (2), the first study reporting detailed inclusion and exclusion criteria for Ni-VATS lobectomy was published by Chen et al. (3) in 2011. Subsequently, other studies generally followed their criteria, with some modifications (8-14). The reported inclusion criteria are generally the same as those for conventional IVATS lobectomy for lung cancer. However, exclusion criteria are more important when considering Ni-VATS lobectomy. Indeed, conditions that may increase the risk for conversion to tracheal intubation and the difficulty of conversion should be taken into account. For example, in a study by Hunget al. involving 1,025 patients who underwent Ni-VATS for lung tumor resections, the authors found that a BMI ≥25 kg/m2 was a risk factor for conversion (15). Those with expected increases in the difficulty of operation, such as extended pleural effusion and coagulopathy, should also be excluded. However, some other feasibility studies have suggested that the non-intubated approach may not be contraindicated for some groups traditionally considered to be high-risk for IVATS, such as patients with impaired lung function (16) and geriatric patients (9).
Anesthetic protocols
In non-intubated VATS, endotracheal intubation and muscle relaxants are waived for thoracoscopic surgery. In addition, the anesthetic and operative protocols vary among different studies and institutes.
The common methods for achieving regional analgesia are TEA and ICNBs. Although TEA was used in initial studies, ICNB provides a simpler, safer, and more effective alternative for intraoperative pain control (17-20). Given the recent development of uniportal VATS that decreases postoperative pain (21,22), the role of TEA may be decreasing. Propofol is most commonly used for sedation, likely due to its fast induction, the ease with which the level of sedation can be altered, and its quick recovery (23). Opioids such as fentanyl or remifentanil are commonly combined with propofol to achieve better analgesia and sedation with a lower dosage, thereby decreasing side effects. Airway and breathing supports can be applied using an oxygen mask or LMA, although the latter may provide better oxygenation and positive pressure ventilation if needed (24,25) and may be a better approach for less experienced surgeons.
Feasibility and safety of Ni-VATS lobectomy for lung cancer
Ni-VATS was initially used in minor operations such as wedge resection for lung nodules or lung volume reduction surgery (1). Due to several concerns such as hypoxemia and hypercapnia induced by prolonged spontaneous single-lung ventilation, coughing induced during manipulation of proximal airways, and lung movement during delicate hilar dissection, it was not until 2011 that Chen and colleagues first reported the comparable safety and short-term results of Ni-VATS lobectomy for the treatment of early NSCLC (3). The studies included in this review also demonstrated the feasibility and safety of the Ni-VATS approach for lobectomy in patients with lung cancer. Induction time, operation time, intraoperative physiological data, and blood loss were similar in the intubated and non-intubated groups. Although one study reported higher peak hypercapnia in the geriatric non-intubated group, the authors believe that this was not clinically significant (9). Surgical techniques, such as the implementation of the single-port approach, have also evolved over time. The feasibility and satisfactory early postoperative outcomes of uniportal Ni-VATS lobectomy have also been reported in other noncomparative studies (13,26,27). Low rates of conversion to tracheal intubation were reported (range, 2.8–10%), and no conversion-related complications were observed. The risk of conversion may also be reduced with careful patient selection and preparation of the medical team, which should include experienced anesthesiologists and surgeons with prior technical practice in minor operations such as wedge resection (15).
Potential benefits of Ni-VATS lobectomy for lung cancer
General intubated anesthesia often causes gastrointestinal dysfunction and increases the risk of postoperative nausea and vomiting (28). This may explain the faster recovery of postoperative oral intake in the Ni-VATS lobectomy group in two studies (3,11). Given that other studies have reported positive findings such as less pleural drainage, fewer complications, and shorter hospital stay in the non-intubated group (10,11), our analysis suggests that non-intubated anesthesia enhances postoperative recovery in patients with lung cancer undergoing VATS lobectomy. Possible mechanisms include avoidance of the side effects of inhalational anesthetics, residual effects of muscle relaxants, possible injury due to endotracheal intubation, and positive pressure-related trauma (29,30). However, due to diversity in anesthetic protocols for Ni-VATS lobectomy, further studies are required to compare different drug choices and airway management techniques, in order to define the best protocol for a non-intubated approach.
Mid-term and long-term oncological outcomes
Since VATS lobectomy is still the standard treatment for lung cancer, the oncological outcomes of Ni-VATS lobectomy are of great importance. Although no perioperative mortality was observed in this review, we are also interested in mid-term and long-term oncological outcomes. Some studies hypothesized that Ni-VATS reduces ventilator-related stress and the need for opioids, which may preserve perioperative anticancer immunosurveillance (29,30). Others also observed a lower impact on postoperative immunological response in awake or Ni-VATS (31-34). In this review, although most studies reported comparable numbers of dissected lymph nodes (3,9,11), AlGhamdi et al. (8) observed significantly fewer dissected lymph nodes in the non-intubated group. It is unclear whether the finding was oncologically significant. Unfortunately, no studies have been able to answer this question. One of our included studies attempted to indirectly compare oncological outcomes with respect to adjuvant chemotherapy compliance and toxicity, proposing that a non-intubated procedure may result in improved adjuvant chemotherapy compliance and lower toxicity rates after lobectomy (10). However, in that study, the comorbidity and pathological staging in the two groups differed, which may have also affected compliance with adjuvant chemotherapy (35,36). Moreover, the potential impact on long-term survival was also unknown.
Limitations
The lack of consistent anesthetic/surgical protocols and outcome reporting is the greatest limitation of this study that prevents detailed analysis. Heterogeneity between the included studies exists in terms of the included population. All studies are retrospective observational studies and therefore suffer from possible bias.
Conclusions
Current evidence consistently demonstrates that Ni-VATS lobectomy for lung cancer may improve postoperative recovery. Potential benefits of Ni-VATS include faster oral intake, less chest tube drainage and duration, and shorter hospital stay. However, there is a paucity of data related to mid-term and long-term oncological outcomes. Studies providing comparable or better oncological results may encourage more thoracic surgeons to perform Ni-VATS lobectomy. Future studies should aim to identify an optimal indication for a specific group who may benefit most from a non-intubated approach, as well as the mid-term and long-term oncological outcomes of Ni-VATS.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Francesco Guerrera, Paolo Albino Ferrari and Roberto Crisci) for the series “Non-Intubated Thoracic Surgery. A Global Perspective” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/vats-21-20
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats-21-20). The series “Non-Intubated Thoracic Surgery. A Global Perspective” was commissioned by the editorial office without any funding or sponsorship. JSC serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from May 2020 to Apr 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761-8. [Crossref] [PubMed]
- Al-Abdullatief M, Wahood A, Al-Shirawi N, et al. Awake anaesthesia for major thoracic surgical procedures: an observational study. Eur J Cardiothorac Surg 2007;32:346-50. [Crossref] [PubMed]
- Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg 2011;254:1038-43. [Crossref] [PubMed]
- Klijian AS, Gibbs M, Andonian NT. AVATS: Awake video assisted thoracic surgery--extended series report. J Cardiothorac Surg 2014;9:149. [Crossref] [PubMed]
- Pompeo E. Awake thoracic surgery—is it worth the trouble? Semin Thorac Cardiovasc Surg 2012;24:106-14. [Crossref] [PubMed]
- Katlic MR. Five hundred seventy-six cases of video-assisted thoracic surgery using local anesthesia and sedation: lessons learned. J Am Coll Surg 2018;226:58-63. [Crossref] [PubMed]
- Zeng X, Zhang Y, Kwong JSW, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta‐analysis, and clinical practice guideline: a systematic review. J Evid Based Med 2015;8:2-10. [Crossref] [PubMed]
- AlGhamdi ZM, Lynhiavu L, Moon YK, et al. Comparison of non-intubated versus intubated video-assisted thoracoscopic lobectomy for lung cancer. J Thorac Dis 2018;10:4236-43. [Crossref] [PubMed]
- Wu CY, Chen JS, Lin YS, et al. Feasibility and safety of nonintubated thoracoscopic lobectomy for geriatric lung cancer patients. Ann Thorac Surg 2013;95:405-411. [Crossref] [PubMed]
- Furák J, Paróczai D, Burián K, et al. Oncological advantage of nonintubated thoracic surgery: Better compliance of adjuvant treatment after lung lobectomy. Thorac Cancer 2020;11:3309-16. [Crossref] [PubMed]
- Liu J, Cui F, Pompeo E, et al. The impact of non-intubated versus intubated anaesthesia on early outcomes of video-assisted thoracoscopic anatomical resection in non-small-cell lung cancer: a propensity score matching analysis. Eur J Cardiothorac Surg 2016;50:920-5. [Crossref] [PubMed]
- Moon Y, AlGhamdi ZM, Jeon J, et al. Non-intubated thoracoscopic surgery: initial experience at a single center. J Thorac Dis 2018;10:3490-8. [Crossref] [PubMed]
- Ahn S, Moon Y, AlGhamdi ZM, et al. Nonintubated uniportal video-assisted thoracoscopic surgery: a single-center experience. Korean J Thorac Cardiovasc Surg 2018;51:344-9. [Crossref] [PubMed]
- Liu J, Cui F, Li S, et al. Nonintubated video-assisted thoracoscopic surgery under epidural anesthesia compared with conventional anesthetic option. Surg Innov 2015;22:123-30. [Crossref] [PubMed]
- Hung WT, Hung MH, Wang ML, et al. Nonintubated thoracoscopic surgery for lung tumor: seven years’ experience with 1025 cases. Ann Thorac Surg 2019;107:1607-12. [Crossref] [PubMed]
- Wang ML, Hung MH, Hsu HH, et al. Non-intubated thoracoscopic surgery for lung cancer in patients with impaired pulmonary function. Ann Transl Med 2019;7:40. [Crossref] [PubMed]
- Hung MH, Chan KC, Liu YJ, et al. Nonintubated thoracoscopic lobectomy for lung cancer using epidural anesthesia and intercostal blockade. Medicine 2015;94:e727 [Crossref] [PubMed]
- Hung MH, Hsu HH, Chan KC, et al. Non-intubated thoracoscopic surgery using internal intercostal nerve block, vagal block and targeted sedation. Eur J Cardiothorac Surg 2014;46:620-5. [Crossref] [PubMed]
- Irons JF, Martinez G. Anaesthetic considerations for non-intubated thoracic surgery. J Vis Surg 2016;2:61. [Crossref] [PubMed]
- Kiss G, Castillo M. Non-intubated anesthesia in thoracic surgery-technical issues. Ann Transl Med 2015;3:109. [PubMed]
- Jutley RS, Khalil MW, Rocco G. Uniportal vs. standard three-port VATS technique for spontaneous pneumothorax: comparison of post-operative pain and residual paraesthesia. Eur J Cardiothorac Surg 2005;28:43-6. [Crossref] [PubMed]
- Bulgarelli Maqueda L, García-Pérez A, Minasyan A, et al. Uniportal VATS for non-small cell lung cancer. Gen Thorac Cardiovasc Surg 2020;68:707-15. [Crossref] [PubMed]
- Höhener D, Blumenthal S, Borgeat A. Sedation and regional anaesthesia in the adult patient. Br J Anaesth 2008;100:8-16. [Crossref] [PubMed]
- Ambrogi MC, Fanucchi O, Gemignani R, et al. Video-assisted thoracoscopic surgery with spontaneous breathing laryngeal mask anesthesia: Preliminary experience. J Thorac Cardiovasc Surg 2012;144:514-5. [Crossref] [PubMed]
- Ambrogi MC, Fanucchi O, Korasidis S, et al. Nonintubated thoracoscopic pulmonary nodule resection under spontaneous breathing anesthesia with laryngeal mask. Innovations (Phila) 2014;9:276-80. [Crossref] [PubMed]
- Furák J, Szabó Z, Horváth T, et al. Non-intubated, uniportal, video assisted thoracic surgery [VATS] lobectomy, as a new procedure in our department. Magyar Sebeszet 2017;70:113-7. [PubMed]
- Gonzalez-Rivas D, Yang Y, Guido W, et al. Non-intubated (tubeless) uniportal video-assisted thoracoscopic lobectomy. Ann Cardiothorac Surg 2016;5:151-3. [Crossref] [PubMed]
- Visser K, Hassink EA, Bonsel GJ, et al. Randomized controlled trial of total intravenous anesthesia with propofol versus inhalation anesthesia with isoflurane-nitrous oxide. Anesthesiology 2001;95:616-26. [Crossref] [PubMed]
- Cheng YJ, Chan KC, Chien CT, et al. Oxidative stress during 1-lung ventilation. J Thorac Cardiovasc Surg 2006;132:513-8. [Crossref] [PubMed]
- Tønnesen E, Höhndorf K, Lerbjerg G, et al. Immunological and hormonal responses to lung surgery during one-lung ventilation. Eur J Anaesthesiol 1993;10:189-95. [PubMed]
- Vanni G, Tacconi F, Sellitri F, et al. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg 2010;90:973-8. [Crossref] [PubMed]
- Tacconi F, Pompeo E, Sellitri F, et al. Surgical stress hormones response is reduced after awake videothoracoscopy. Interact Cardiovasc Thorac Surg 2010;10:666-71. [Crossref] [PubMed]
- Mineo TC, Ambrogi V. Immune effects after uniportal nonintubated video-thoracoscopic operations. Video-assisted Thorac Surg 2018;3:4. [Crossref]
- Oh SA, Jeon J, Hwang W. Comparison of postoperative immunological changes between intubated and non-intubated patients during lung cancer resection surgery European Respiratory Journal 2019;54:PA2215 [Crossref]
- Landrum MB, Keating NL, Lamont EB, et al. Reasons for underuse of recommended therapies for colorectal and lung cancer in the Veterans Health Administration. Cancer 2012;118:3345-55. [Crossref] [PubMed]
- Bao H, Xu N, Li Z, et al. Effect of laparoscopic gastrectomy on compliance with adjuvant chemotherapy in patients with gastric cancer. Medicine 2017;96:e6839 [Crossref] [PubMed]
Cite this article as: Chuang JH, Hung WT, Chen JS. Narrative review of non-intubated video-assisted thoracic surgery (Ni-VATS) for lung cancer: a focus on lobectomy. Video-assist Thorac Surg 2021;6:25.


