
The anesthesiologist perspective
Introduction
We are facing an era of great challenges and opportunities. Since new requests arise, new answers should be offered to our patients, especially to the more fragile ones.
Cooperation between anesthesiologists and thoracic surgeons plays a pivotal role in successfully managing difficult procedures in vulnerable patients. Non-Intubated Thoracic Surgery (NITS) is among the techniques found effective to handle this population, offering a number of surgical opportunities for patients who are too risky for general anesthesia (GA): one-lung ventilation under spontaneous breathing allows maintaining a better match of ventilation and perfusion; respiratory efficiency can be guaranteed by a preserved diaphragmatic function, intubation-related trauma and mechanical ventilation are avoided, and no residual neuromuscular blockage problems occur.
Nevertheless, the intraoperative management, as well as management in critical situations, is quite different during NITS compared to the standard procedures performed under GA.
We will briefly discuss some key topics, starting from the Anesthesiologist’s Perspective, while keeping in mind that a multidisciplinary approach is essential for safe and effective management.
Preoperative phase
In the preoperative phase patient counseling and engagement are crucial. A proper selection for patients scheduled for NITS, e.g., avoiding the most anxious or the least cooperative patients, is essential. After a careful and multidisciplinary selection, patients must be informed and motivated by providing them a complete description of the procedure, which may be useful to be repeated more than once.
There are no anesthesiologic limits to the surgical procedures that can be performed with this approach. There is evidence of management of pleural effusion, bullectomy for spontaneous pneumothorax and giant bullous disease, resection of pulmonary nodules, lung metastases and lung cancer surgery, pleural decortication, lung volume reduction surgery, thymectomy, thoracotomy, pneumonectomy, and even tracheal surgery (Table 1).

Full table
Although there is no consensus regarding exclusion criteria (Table 1), expert opinions suggest comorbidities such as obesity and smoking as relative contraindications. A consensus of experts in 2019 outlined possible inclusion and exclusion criteria specifically to shed light on the execution of NITS procedures (8).
It is therefore clear that there is considerable confusion about the eligibility criteria of the patient candidate for NITS. However, it is shared that poor performance status (e.g., ASA, NYHA, MET), as well as the presence of impaired preoperative lung function or significant arterial blood gases alterations contraindicate both GA and NITS.
NITS—Physiological considerations
Performing thoracic surgery in non-mechanically ventilated patients requires extremely careful anesthetic management basically due to two physiological phenomena: Pendelluft and mediastinal shift (9). During the surgery, the non-operated hemithorax receives a flow of inspiratory gas not only from the trachea, but also from the operated lung, which collapses due to surgical pneumothorax. This implies that during inspiration the non-operated lung, called dependent lung (DL), expands and, conversely, the operated lung, known as non-dependent lung (NDL), collapses. The resulting movement is called the ‘pendular’ movement of the lung, which occurs in both the inspiratory and expiratory phases. Similar behavior has the mediastinum with consequent dislocation of the structures contained in it (10). Minimally invasive procedures such as Video-Assisted Thoracic Surgery (VATS) allow a minimization of these effects, thus making NITS a safer technique especially in high-risk patients (11).
It should also be considered that thoracic surgery is performed in lateral decubitus, this means that the DL receives most of the ventilation in the lower portions and the NDL in the upper ones. Lateral decubitus also distributes the perfusion of the lung parenchyma in a similar way by the direct gravity’s effect. As a result, the DL gets about 10% more cardiac output (12).
Summarizing, the lateral decubitus promotes both ventilation and perfusion of the DL, with a with a ventilation/perfusion (V/Q) ratio similar to that found in standing position. Hence some simple practical considerations: first of all, the fact that oxygenation in these patients is usually quite manageable with modest increase in the inspired fraction of oxygen (FiO2). Secondly, the crucial moment for the onset of dyspnea and discomfort is when the surgical pneumothorax is realized. As a result, in this particular phase, it may be useful to achieve a deeper sedation level in order to prevent subjective symptoms onset. In conclusion, lateral decubitus is the best position for the patient’s respiratory performance during surgery, despite the fact that this may reserve technical difficulties in case of advanced airway management.
Intraoperative phase
There is currently no consensus on intraoperative management, probably because of the wide variability within different centers’ strategies (13).
Benzodiazepines, propofol, ketamine, dexmedetomidine with or without opioids sedation could be used to have a cooperative and calm patient. The combined use of these drugs often allows the achievement of a better effect while reducing overdose risks.
Given that a stationary operating field is necessary in order to guarantee the success of the NITS procedures while reducing the risk of postoperative air leaks, it may be useful to manage the cough reflex with the use of lignocaine aerosol, with the administration of opioids or with vague nerve block. The use of local anesthetic spray of the pleura has also been described in the literature (14).
Although the efficacy of these techniques has not yet been confirmed in the context of NITS by randomized clinical trials, locoregional anesthesia techniques can be used to achieve an appropriate analgesic effect.
In a meta-analysis conducted by Deng et al. it has been reported that regional anesthesia techniques such as Thoracic Epidural Anesthesia (TEA), intercostal nerve blocks, and Paravertebral Blocks (PVB) can be effective, especially when combined with mild sedation (15). Recently, new techniques have been reported in the context of thoracic surgery such as Erector Spinae Plane Block (ESPB) and Serratus Plane Block (SPB) associated or not with Pectoral Nerves Blocks (PECS I or II) (16).
In addition to new anesthesia techniques, it is noteworthy that even new local anesthetics are beginning to be used in thoracic surgery. Liposomal bupivacaine, which use is still off-label for applications such as TEA and blocks including ESPB, PVB, and intercostal nerve blocks, seems to be effective even in the context of thoracic surgery (17). It is therefore desirable to use it, also in the context of NITS as soon as the indication is confirmed.
If the surgical procedures are not complex, NITS can be accomplished with invasive airway management. An optimal oxygenation level can be in fact easily achieved with simple oxygen masks even in compromised patients. In case of severe hypoxemia, it may be useful to inflate the NDL by a suctioning maneuver from the operative field and/or to reduce the depth of sedation. Only in more complex cases it may be necessary to convert NITS into GA by orotracheal intubation either in lateral decubitus or after supination (18). The possible use of a laryngeal mask, even in conjunction with deep sedation, is not configured as a conversion from NITS to GA.
However, everything should be prepared for a possible conversion of the intervention into GA (19). Regarding the management of difficult airways, a dedicated pathway should be developed possibly in a Crises Resource Management (CRM) context.
Particular attention must be paid to the elimination of carbon dioxide which can be a critical problem even if hypercapnia is normally controlled (Figure 1) with an adequate level of sedation and a precise titration of opioids.
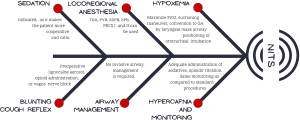
NITS is most frequently converted into GA for the following reasons: major bleeding, severe pleural adhesions, severe hypercapnia and acidosis, hypotension, hypoxemia, intractable arrhythmias, right ventricle failure, persistent cough that hinders the performance of the surgery, excessive diaphragm movement, inability to collapse the lung (20). However, it should be noted that the conversion rate, as also reported by Chen et al., is ranging around 4.9% (6).
Postoperative phase
According to the literature, postoperative complications, length of hospital stays, and consequently medical costs are better with NITS compared to VATS under GA (21). In addition, it has been reported shorter duration of anesthesia, reduced surgical and total time in the operating room, as well as better satisfaction of patients, less need of nurse care, and lower overall intake of postoperative medications. Recent studies measured lower levels of inflammatory cytokines, or lymphocytic response, and reduced blood levels of stress hormones during NITS (22).
The postoperative phase in patients undergoing NITS is therefore faster and qualitatively better than in those receiving GA. This has been confirmed by a study conducted by Liu et al. (23) reporting that NITS/VATS biopsies in Interstitial Lung Disease have less postoperative morbidity, less Intensive Care Unit (ICU) admissions, and hospital stays three times lower (13).
Crisis resource management (CRM)
CRM include skills that fall within different clinical fields, including emergency medicine, and include non-technical skills necessary for effective team management in crisis situations. CRM’s main elements are awareness of the surrounding environment and available resources, having a plan in advance and an early call for help, exercising leadership and followership, distributing tasks in a prepared manner, mobilizing all available resources, communicating effectively. It is also necessary to prevent any kind of error, always using double-check, re-evaluating the situation and properly evaluating priorities (19).
NITS procedures are a challenge for all OR staff. Complications that occur may or may not be predictable. In these circumstances, prompt treatment is crucial, and this can only be achieved through a team-build approach where preparation, coordination, and strong and consistent leadership are mandatory. Since these are risky procedures, the patient should be the first of the day and it is useful to have a checklist available, similar to the one proposed by Navarro-Martínez et al. (19).
Future perspective
The NITS technique is still little known and applied. Further studies are ongoing to evaluate the efficacy of new methods to improve oxygenation in case of hypoxemia, such as high flow nasal cannulas or trans-nasal humidified rapid-insufflation ventilator exchange, possibly improving oxygenation and carbon dioxide clearance or studies evaluating the effect of vagal cervical blockage on cough reflex.
The issue of the effect of muscle relaxants on diaphragmatic function is also of particular relevance given that a reduction in functional residual capacity (FRC), possibly related to the use of anesthesia and muscle relaxants has been reported.
Among the most interesting monitoring systems recently introduced in anesthetic practice there is certainly the ultrasound evaluation of the diaphragm which is the subject of a growing literature especially in the ICU field (24).
But the attention towards diaphragmatic activity is also growing in postoperative care context. Spadaro et al. have indeed reported an increased risk of pulmonary complications in patients with impaired diaphragmatic function after thoracic surgery (25). In the same study, the relationship between diaphragmatic dysfunction and postoperative pulmonary complications in thoracic surgery has been evaluated. Comparing data on ultrasound measured diaphragmatic function in patients undergoing thoracotomy or VATS, Spadaro et al. found that VATS patients had a lower reduction in the diaphragmatic excursion and therefore fewer pulmonary complications (24).
Based on preliminary data from an ongoing study performed in our center (Registered on Clinicaltrials.gov, NCT04700943), there is less inhibition of diaphragmatic function during NITS compared to GA.
Conclusions
From the Anesthesiologist perspective, NITS is a high-intensity procedure, which according to the most recent scientific literature appear to be cost-effective. Based on our single centers experience it is possible to consider NITS more as an energy and time investment rather than a dangerous and complicated procedure. Future research in this field should be conducted in order to be tailored to the patient, ensuring a personalized approach based on patient characteristics and specific requirements of the surgical procedure.
Acknowledgments
We are in debt to all the physicians and nurses who contributed to commute the risky NITS mule track into a safe highway for all patients.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Francesco Guerrera, Paolo Albino Ferrari and Roberto Crisci) for the series “Non-Intubated Thoracic Surgery. A Global Perspective” published in Video-Assisted Thoracic Surgery. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats-21-2). The series “Non-Intubated Thoracic Surgery. A Global Perspective” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liu J, Li S, Shen J, et al. Non-intubated resection and reconstruction of trachea for the treatment of a mass in the upper trachea. J Thorac Dis 2016;8:594-9. Erratum in: J Thorac Dis 2016;8:E473. [Crossref] [PubMed]
- Hung WT, Liao HC, Cheng YJ, et al. Nonintubated Thoracoscopic Pneumonectomy for Bullous Emphysema. Ann Thorac Surg 2016;102:e353-5. [Crossref] [PubMed]
- Liu Z, Yang R, Sun Y. Non-intubated subxiphoid uniportal video-assisted thoracoscopic thymectomy. Interact Cardiovasc Thorac Surg 2019;29:742-5. [Crossref] [PubMed]
- Jiang L, Liu J, Shao W, et al. Non-intubated subxiphoid uniportal video-assisted thoracoscopic thymectomy using glasses-free 3D vision. J Thorac Dis 2016;8:E1602-4. [Crossref] [PubMed]
- Furák J, Szabó Z, Tánczos T, et al. Conversion method to manage surgical difficulties in non-intubated uniportal video-assisted thoracic surgery for major lung resection: simple thoracotomy without intubation. J Thorac Dis 2020;12:2061-9. [Crossref] [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis 2012;4:347-51. [PubMed]
- Okuda K, Nakanishi R. The non-intubated anesthesia for airway surgery. J Thorac Dis 2016;8:3414-9. [Crossref] [PubMed]
- He J, Liu J, Zhu C, et al. Expert consensus on tubeless video-assisted thoracoscopic surgery (Guangzhou). J Thorac Dis 2019;11:4101-8. [Crossref] [PubMed]
- Maloney JV Jr, Schmutzer KJ, Raschke E. Paradoxical respiration and “pendelluft”. J Thorac Cardiovasc Surg 1961;41:291-8. [Crossref] [PubMed]
- Slinger P. editor. Principles and Practice of Anesthesia for Thoracic Surgery. Springer, 2019.
- Tacconi F, Pompeo E, Fabbi E, Mineo TC. Awake video-assisted pleural decortication for empyema thoracis. Eur J Cardiothorac Surg 2010;37:594-601. [Crossref] [PubMed]
- Wulff KE, Aulin I. The regional lung function in the lateral decubitus position during anesthesia and operation. Acta Anaesthesiol Scand 1972;16:195-205. [Crossref] [PubMed]
- Pompeo E, Sorge R, Akopov A, et al. Non-intubated thoracic surgery-A survey from the European Society of Thoracic Surgeons. Ann Transl Med 2015;3:37. [PubMed]
- Jiang L, Liu J, Gonzalez-Rivas D, et al. Thoracoscopic surgery for tracheal and carinal resection and reconstruction under spontaneous ventilation. J Thorac Cardiovasc Surg 2018;155:2746-54. [Crossref] [PubMed]
- Deng HY, Zhu ZJ, Wang YC, et al. Non-intubated video-assisted thoracoscopic surgery under loco-regional anaesthesia for thoracic surgery: a meta-analysis. Interact Cardiovasc Thorac Surg 2016;23:31-40. [Crossref] [PubMed]
- Pirsaharkhiz N, Comolli K, Fujiwara W, et al. Utility of erector spinae plane block in thoracic surgery. J Cardiothorac Surg 2020;15:91. [Crossref] [PubMed]
- Campos JH, Seering M, Peacher D. Is the Role of Liposomal Bupivacaine the Future of Analgesia for Thoracic Surgery? An Update and Review. J Cardiothorac Vasc Anesth 2020;34:3093-103. [Crossref] [PubMed]
- Coley EL, Irons JF. Non-intubated anesthetic techniques for thoracic surgery. Video-assist Thorac Surg 2017;2:69. [Crossref]
- Navarro-Martínez J, Gálvez C, Rivera-Cogollos MJ, et al. Intraoperative crisis resource management during a non-intubated video-assisted thoracoscopic surgery. Ann Transl Med 2015;3:111. [PubMed]
- Hung MH, Wang YP. Preventing Hypoxemia During Nonintubated Thoracoscopic Surgery. Ann Thorac Surg 2020;110:747-8. [Crossref] [PubMed]
- Pompeo E, Tacconi F, Mineo TC. Comparative results of non-resectional lung volume reduction performed by awake or non-awake anesthesia. Eur J Cardiothorac Surg 2011;39:e51-8. [Crossref] [PubMed]
- Yu MG, Jing R, Mo YJ, et al. Non-intubated anesthesia in patients undergoing video-assisted thoracoscopic surgery: A systematic review and meta-analysis. PLoS One 2019;14:e0224737 [Crossref] [PubMed]
- Liu CY, Hsu PK, Chien HC, et al. Tubeless single-port thoracoscopic sublobar resection: indication and safety. J Thorac Dis 2018;10:3729-37. [Crossref] [PubMed]
- Spadaro S, Grasso S, Dres M, et al. Point of Care Ultrasound to Identify Diaphragmatic Dysfunction after Thoracic Surgery. Anesthesiology 2019;131:266-78. [Crossref] [PubMed]
- Tuinman PR, Jonkman AH, Dres M, et al. Respiratory muscle ultrasonography: methodology, basic and advanced principles and clinical applications in ICU and ED patients—a narrative review. Intensive Care Med 2020;46:594-605. [Crossref] [PubMed]
Cite this article as: Rosboch GL, Ceraolo E, Balzani E, Piccioni F, Brazzi L. The anesthesiologist perspective. Video-assist Thorac Surg 2021;6:21.


