
Algorithm for the pulmonary metastasectomy based on number of metastases and histology
Introduction
Video-assisted thoracic surgery (VATS) is being increasingly used for treatment of lung metastases. In this paper, we try to define the practicability and feasibility of the VATS and open approaches for lung metastasectomy. Thereby comparing the two approaches and indicating when which one is more advisable. From an academic point of view, the differences between VATS and conventional thoracotomy have been listed below (Table 1).
Table 1
| Characteristics | Conventional thoracotomy | VATS |
|---|---|---|
| Operative access | Large incision, rib spreading | Small incision, muscle spreading |
| Pain | More, longer periods | Less, shorter periods |
| Post-thoracotomy pain syndrome | High risk | Rare, low risk |
| Length of hospital stay | Longer | Shorter, enhanced recovery protocols |
| Postoperative complications | Increased incidence | Less |
| Metastasis detection | Better, manual palpation whole lung | Inadequate palpation |
| Resection technique | Laser, cautery, staples | Staples (laser, cautery) |
| Resected tissue | Precision resection possible | Various margin lengths |
| Repeat operations | Excessive scaring and adhesions | Rather easy, lesser adhesions |
| Oncologic outcome | Many retrospective large series | Few reports, small series |
| Metastasis recurrences | Depending on histology and number of metastasis | “Overseen lesions”? Recurrence at the staple line? |
VATS, video-assisted thoracic surgery.
A clear disadvantage of the VATS approach is the inability to perform a systematic manual palpation of the lung and therefore small nodules may be missed leading to an incomplete resection (R2) (1). Completeness of resection after metastasectomy is the most important prognostic factor described in the largest ever published metastasectomy collective (2). The lung specimens resected with staplers through the VATS approach are predominantly linear with greater safety margins at the periphery and lesser margins deeper in the tissue usually directly under the nodule. This results in a greater tissue loss, as compared to that after a precision laser or cautery enucleation (Video 1) and might influence local recurrence rates in certain tumor types. These may in turn impact the functional and oncologic outcome (3). Hence, the number of nodules, the size and location in the lung and the risk for local recurrence in case of small safety margins impacts the decision for the surgical approach.
Methods
A selective literature review has been undertaken by the authors to create a clinical guideline, and to shed light upon the aforementioned issues. When available, systematic reviews were preferred to clarify the facts. Finally, our personal experiences with metastasis surgery have been added to this review.
Results
Open thoracotomy versus VATS
Every pulmonary metastasectomy needs surgical access through the thoracic wall, this usually being an incision through the intercostal space. It is easy to understand that the VATS approach being associated with smaller incisions, no retraction of the ribs and less scar formation has some clear advantages over conventional thoracotomies. This difference has been studied thoroughly in regard to the treatment of non-small cell lung cancer (NSCLC). Improved early outcome, decreased pain, shorter length of stay, fewer complications and a more rapid return to function are the parameters of VATS lobectomy that are mentioned in the National Comprehensive Cancer Network (NCCN) 2020 guidelines (4-7). It can be stated that a smaller incision guaranteed by VATS is superior as compared to open thoracotomy for the patient receiving lobectomy. To our belief, this is also true for lobectomy or segmentectomy in metastasectomy patients and lymph node dissection can also be easily performed by VATS (Figure 1).

VATS and number of metastases
As the benefits of VATS rest upon small incisions and no rib spreading, superficial lung palpation is possible “up till the reaches of the index finger”. Bimanual palpation or at least bidigital palpation of intermediate or central is not possible without rib spreading. Therefore, three questions present themselves in concern to metastasectomy by VATS approach: is there a greater probability of undetected additional lesions, localization of the nodules detected preoperatively on the computed tomography (CT) in the lung parenchyma and if detected, is a resection using staples possible?
Undetected additional lesions: preoperative imaging
A retrospective study including 521 patients with colorectal cancer (CRC) lung metastases demonstrated only a moderate concordance between both CT scan (kappa index: 0.42) and fluorodeoxyglucose (FDG)-positron emission tomography (PET) (kappa index: 0.42) findings and the histologically proven number of metastases. Only 61.7% and 61.8% of histologically diagnosed metastases were correctly identified with the CT and FDG-PET scans, respectively (8). In a prospective trial, identification of lung metastases was compared between radiologists and a cloud cased computer-aided detection (CAD) system validated by experienced radiologists. From 225 patients, 75 had a total of 215 nodules. The sensitivity to detect lesions ≥3 mm was significantly higher using CAD (65% vs. 88%, P<0.01). Thus, the authors concluded that CAD-assisted reading improves nodules detection in patients with extrathoracic malignancies (9). Another recent prospective trial demonstrated that the use of CAD increased the sensitivity to detect lung metastases from 67.5% for all lesions (87.4% for metastases) to 77.9% (92.7% for metastases) (P<0.001). Nonetheless, 143 additional lesions (19 metastases) were detected during surgery (10). Park et al. (11) evaluated nodule detection of four readers who interpreted 1-mm sections of multidetector CT (MDCT), their detection rates increased from 91%, 88%, 87%, and 86% to 94%, 96%, 91%, and 92%, respectively, when the readers evaluated 5-mm maximum intensity projection (MIP) technique reconstructed at 1-mm intervals; the sensitivity change was significant for 3 of the 4 readers (11).
Undetected additional lesions: histology
It is a matter of insight from daily practice, that certain tumors arise with multiple metastases and others are more likely to manifest as an oligometastatic disease, implying the influence of various histological types on the presentation morphology.
Kang et al. evaluated 27 patients with 1 mm thin-section 16-channel multi-detector row CT before pulmonary metastasectomy (12). A total of 117 nodules were detected preoperatively and 198 nodules were resected. A total of 101 nodules were pathologically confirmed to be metastatic nodules. The sensitivity, specificity, positive predictive value, and negative predictive value were lowest in the osteosarcoma group. Thin slice CT scan achieved 100% sensitivity when nodule size was over 5 mm and less than five nodules were detected in the non-osteosarcoma group (12).
Macherey et al. (1) analyzed 18 studies on 1,472 patients with lung metastases having 1,630 pulmonary metastasectomies between 1990 and 2014 with 30% of patients undergoing VATS. The sensitivity of preoperative CT scans detecting lung metastases increased with decreasing slice thickness and was dependent on histology. The sensitivity was 60–97% for epithelial tumors and higher when CT slices were thin (89% for 3-mm slice thickness). The specificity was highest (93%) for osteosarcoma compared to 54–63% for other histologies. More occult metastases (non-imaged) were found during operation, when the target lesions were small and the preoperative number of lesions were 2 or more. Not every lung nodule is a metastasis and up to 50% of non-imaged nodules palpated and resected during the operation were benign leading to a rate of false-positive findings between 13% and 48.5%. The authors state that open approach is superior in detecting occult nodules but their prognostic significance remains unclear (1).
Undetected additional lesions: number of metastases
In a later study 120 operations for pulmonary metastases with open palpation of the lungs were performed in 91 patients with CRC. The number of removed metastases was consistent with preoperative CT scan in 53.3% and additional metastatic tumors were found in 26.7% (13) (Table 2).
Table 2
| No. of metastasis on CT scan | N (%) | Additional benign nodules, n (%) | Additional malignant nodules, n (%) | Sensitivity of CT scan (%) (detection of malignant nodules) |
|---|---|---|---|---|
| 1 | 65 (54.2) | 18 (27.7) | 6 (9.2) | 90.80% |
| 2 | 34 (28.3) | 6 (17.6) | 10 (29.4) | 70.60% |
| 3 | 9 (7.5) | 0 (0) | 4 (44.4) | 55.60% |
| ≥4 | 12 (10.0) | 0 (0) | 12 (100.0) | 0% |
CT, computed tomography.
Metastases count showed a trend of negative association with additional benign nodules whilst there was a significant positive correlation with additional malignant nodules (P=0.051 and P<0.001, respectively).
The sensitivity of helical computed tomography in the detection of CRC metastases ranged from 35.5% to 95.5%. Unilateral solitary lesions were found with additional malignant lesions in less than 5% (P=0.023) (Figure 1) and increasing numbers of metastases were associated with increasing numbers of additional undetected metastases (Figure 2), always (100%) when 4 or more lesions were present (13).

Marron et al. (14) using the Spanish national registry to look for relationship between chest CT and pathologic nodule counts in in 404 patients having open pulmonary metastasectomy for CRC lung metastases. Unilateral involvement was found in 345 (85%) and single nodule in 253 (63%) patients. The radiologic and malignant pathologic findings were concordant in 316 (78%) patients. Independent predictors of discordance were bilateral involvement and greater number of metastases. Imaging and operative agreement of metastasis counts was 95% for single lesions and 50% with more than one radiologic nodule. Significant disagreements were found for advanced-stage CRC at initial diagnosis, smaller nodules (13.5 vs. 18.4 mm), simultaneous liver metastases (P=0.014), bilateral metastases (P<0.001), increasing numbers of nodules (P<0.001). Every additional nodule increased the discrepancies with an odds ratio of 6.17 and every decreasing millimeter in the size of the metastases increased discrepancies by 5% (14).
Nodule identification in the parenchyma
Using VATS approach requires the intraoperative identification of the nodules by visualization, fingertip palpation (Figure 1) or with a touch-bar. This is not always successful.
A comprehensive study by Eckardt et al. (15) about the completeness of pulmonary metastasectomy by VATS was undertaken in 89 patients with a maximum of 3 ipsilateral, peripheral lung nodules on the CT scan and were explored by 3-port VATS from one surgical team. Every identified nodule was described and documented. After that, a different surgical team took over and performed an open thoracotomy with bimanual palpation and wedge resection of all the nodules using staples. One hundred and forty nodules were identified preoperatively on CT scan, and 122 of these (87%) were documented by VATS with additional 67 nodules removed by open surgery. Of these additional nodules 33% were metastases, 64% were benign lesions and 3% were unexpected lung cancers. The authors concluded that VATS approach is not safe when the primary intention is to clear all pulmonary metastases during surgery. But they also pointed out that VATS is easier to be repeated in case of recurrent metastases (15). To aid in easy identification of intrapulmonary nodules, various modalities have been described, such as hookwire, dye, contrast medium injection, radiotracer-guided or CT-guided localization and microcoil and fiducial marker placement (16).
Survival after VATS metastasectomy
Murakawa et al. analyzed a retrospective multi-institutional database of 1,047 patients after CRC lung metastasectomy between 1999 and 2014. Using propensity score matching, the thoracoscopy group had a better survival rate than the open group. The difference was largest when thoracoscopy was combined with anatomical resection in a predictive model (17).
In a meta-analysis on the different outcomes of open versus VATS metastasectomy comprised of eight retrospective studies including 822 patients (485 after open thoracotomy and 337 after VATS) were put together for a pooled analysis. Compared to open metastasectomy, VATS was associated with a hazard ratio (HR) of 0.69 (P=0.002). No difference was found for recurrence free survival (RFS) (HR of 0.86). Additionally, OS and RFS had no difference between VATS and open surgery in the subgroup of CRC lung metastases (18).
Mode of resection dependent on histology
CRC lung metastases have a high rate of interstitial spread and along with epithelial tumor, lung metastases show free floating tumor cell clusters in the neighboring alveolar spaces (ASFCs). Sarcoma lung metastases, on the other hand, show the highest incidence of pleural infiltration and tend to grow along the connective tissue borders (19). The risk of pleural invasion, lymphangitic spread, interstitial growth, perivascular growth and peribronchial growth increases with increase in the size of the metastases. Factors that determine local recurrence are pleural infiltration, interstitial growth, size of the metastasis, safety margins and ASFCs. Even though the results weren’t significant, there is still a trend that metastases with smooth surfaces tend to be less infiltrative and hence have lesser recurrences (19). It has been shown that segmentectomy offered lower local recurrence as compared to wedge resection for CRC lung metastases (20). Larger metastatic lesions measuring >1.4 cm have been proven to benefit more from anatomical resections (20,21). There have also been reports showing better OS and lower recurrences at the resection margin after anatomical resections in patients with KRAS-mutated CRC lung metastases (22). The overall local recurrence rates after wedge resection of single CRC lung metastasis were 12/65 (18.5%), analyzed in a retrospective series (23). This is a high rate compared to anatomical resections for stage I NSCLC.
Discussion
VATS has shown some clear advantages over conventional thoracotomy, such as less pain and less analgesia requirements, shorter duration of drainage, shorter hospital stay, less complications and better physical performance on short-term follow-up (5,6). Therefore, minimally invasive approach should be the preferred approach whenever possible and beneficial for the patient. VATS will be oncologically beneficial compared to open thoracotomy when completeness of resection can be achieved and safety margins are not jeopardized. VATS metastasectomy has demonstrated encouraging results by showing good survival rates (24). Completeness of resection is the most important prognostic factor (2) and not only includes the complete removal of the main lesion, but also the removal of all additional lesions that might not be detected in a CT scan. Hence, it is obligatory to include risk factors such as the presence of unexpected additional metastases and risk for incomplete resection or insufficient safety margins into our operation planning.
The reliability of preoperative CT scan to detect all metastases has increased in the last twenty years. There have been many innovations, from MDCT scans to helical data acquisition and decreasing slice thickness. Minimum requirements for preoperative CT imaging is a helical CT scan with 3- to 5-mm reconstruction thickness or a volumetric thin section scanning performed up to 4 weeks before pulmonary metastasectomy (25). The widespread availability of MDCT scanners provides the opportunity to examine thin-section (1 mm) CT scans. MIP techniques were shown to improve the visualization of small nodules and is currently used by many radiologists (26). The average sensitivity of nodule detection using 1-mm section increased from 88% to 93.25% with the additional use of MIP technique (11). A further increase of the detection of small nodules was shown with CAD techniques (9-11).
Number of identified metastases with preoperative imaging
It is not surprising that increasing numbers of identified metastases are associated with increasing risk for undetected additional metastases. Marron et al. (14) found an accurate correlation of preoperative imaging with intraoperative findings for single nodules, but found discrepancies in half of the patients with multiple nodules (14). Others as well-found high correlation between preoperative imaging and intraoperative findings when only one lesion was detected (13,27). The percentage of intraoperatively detected unexpected malignant nodules increased from 9% to 30% and 45% when 1, 2 and 3 nodules were identified preoperatively. This implies that more than 2 nodules should be resected with open thoracotomy and manual palpation. In 2010 the European Society of Thoracic Surgeons (ESTS) working group on pulmonary metastasectomy concluded, that at that time, there was no alternative to palpation in every metastasectomy procedure (28). Today, we think, that at least single metastasis evaluated with thin section CT scans may be operated by VATS.
Size of the preoperatively identified metastases
In a study by Kang et al. (12) only 12 of 32 metastatic nodules from osteosarcoma were detected by preoperative multi-detector CT imaging. This was not only a question of histology, but a question of small sized nodules less than 3 mm which are quite common in osteosarcoma (12). These nodules are easy to palpate because they most often are of firm consistency but may be missed by VATS if not detected with CT. On the other hand, no additional nodules were found in non-osteosarcoma patients when less than 5 nodules were detected and all were over 5 mm and disease-free interval was over 24 months. In this situation the CT sensitivity was 100% (12).
Histology of the primary tumor
The probability of preoperatively undetected lesions is higher for mesenchymal (41%) tumors than for epithelial tumors (28%) (29). Metastases with aggressive growth patterns need greater resection margins (>7 mm) to prevent or reduce the risk of local recurrences, making these lesions ideal candidates for anatomical resections. On the other hand, metastases with a smooth surface could be removed with minimal safety margins (30).
Since the wedge resections cannot guarantee an adequate safety margin the deeper the lung metastases are located, anatomical resections are clearly a better choice here (23). For multiple metastases, wedge resections or enucleations with the added benefit of lung parenchyma conservation are more ideal.
The superiority of metastasectomy through anatomic resections like segmentectomy can be attributed to the fact that these procedures offer a better removal of intrapulmonary lymph structures and blood vessels as compared to wedge resection (30). Wedge resections for CRC lung metastases with a median size of 1.1 cm were associated with an intolerable rate of 18% local recurrences, raising the question of anatomical resection for single metastases, at least when they are located deeper in the parenchyma (23).
Intraoperative nodule detection
The use of various localization methods for intrapulmonary nodules depends on a number of factors, such as the treating doctor, availability of resources, number and location of nodules, patient comorbidities, etc. (16). The creation of a gold standard is therefore not possible and the ultimate decision lies with the treating doctor. These modalities can definitely increase the usability of VATS in metastases resections but cannot unfortunately replace a bimanual palpation.
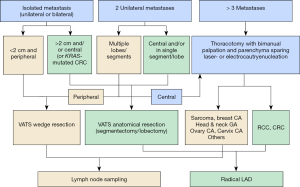
VATS wedge resection is the recommended approach for isolated unilateral or bilateral metastases with peripherally located nodules (<2 cm) as well as for peripherally located two unilateral metastases in multiple segments/lobes. VATS anatomical resection is to be favored in isolated metastases (>2 cm) or centrally located or KRAS-mutated CRC and in two unilateral metastases with central location in a single segment/lobe. In case of more than 3 metastases or central location of two metastases in multiple segments/lobes, a thoracotomy with bimanual palpation and a parenchyma sparing laser (Video 1) or electrocautery enucleation is the approach of choice. A radical lymphadenectomy is recommended in all anatomical resections as well as metastasis of renal cell carcinoma (RCC) and CRC. In all other patient groups, lymph node sampling is sufficient (Figure 3). The long-term outcomes after metastasectomy equally depend on the morphology and histology of the metastasis as well as the surgical skills and the type of operative resection approach employed. It is vital for an optimal outcome to individualize a concept for the patient based on the aforementioned characteristics.
Conclusions
VATS has an increasingly important role in lung metastases surgery. Minimally invasive approach allows enhanced recovery protocols and is associated with less pain, less complications, better functional outcome and shorter hospital length of stay. Its usefulness however, is limited when multiple or deeper parenchymal lesions have to be resected (Figure 2). Pulmonary metastasectomy by open thoracotomy is the standard procedure for 2 or more ipsilateral lesions, allowing manual palpation of the entire lung and thereby identifying additional undetected lesions. VATS approach is an alternative in special situations and should be preceded by a detailed radiological evaluation and when available using techniques such as MIP and CAD to increase detection sensitivity.
Acknowledgments
We would like to thank Dr. Ka-Won Noh for her helpful comments.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marcello Migliore and Michel Gonzalez) for the series “VATS in Lung Metastasectomy” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats-2020-lm-09). The series “VATS in Lung Metastasectomy” was commissioned by the editorial office without any funding or sponsorship. Both authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Macherey S, Doerr F, Heldwein M, et al. Is manual palpation of the lung necessary in patients undergoing pulmonary metastasectomy? Interact Cardiovasc Thorac Surg 2016;22:351-9. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Petrella F, Chieco P, Solli P, et al. Which factors affect pulmonary function after lung metastasectomy? Eur J Cardiothorac Surg 2009;35:792-6. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Non-small Cell Lunge Cancer. Version 2. 2021. Available online: www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Cheng D, Downey RJ, Kernstine K, et al. Video-assisted thoracic surgery in lung cancer resection: a meta-analysis and systematic review of controlled trials. Innovations (Phila) 2007;2:261-92. [Crossref] [PubMed]
- Yang CJ, Kumar A, Klapper JA, et al. A National Analysis of Long-term Survival following Thoracoscopic Versus Open Lobectomy for Stage I Non-small-cell Lung Cancer. Ann Surg 2019;269:163-71. [Crossref] [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8. [Crossref] [PubMed]
- Guerrera F, Renaud S, Schaeffer M, et al. Low Accuracy of Computed Tomography and Positron Emission Tomography to Detect Lung and Lymph Node Metastases of Colorectal Cancer. Ann Thorac Surg 2017;104:1194-9. [Crossref] [PubMed]
- Vassallo L, Traverso A, Agnello M, et al. A cloud-based computer-aided detection system improves identification of lung nodules on computed tomography scans of patients with extra-thoracic malignancies. Eur Radiol 2019;29:144-52. [Crossref] [PubMed]
- Meybaum C, Graff M, Fallenberg EM, et al. Contribution of CAD to the Sensitivity for Detecting Lung Metastases on Thin-Section CT - A Prospective Study with Surgical and Histopathological Correlation. Beitrag der computerassistierten Detektion (CAD) zur Sensitivität der präoperativen Lokalisation von Lungenmetastasen im Dünnschicht-CT – prospektive Studie mit chirurgischer und histopathologischer Korrelation. Rofo 2020;192:65-73. [Crossref] [PubMed]
- Park EA, Goo JM, Lee JW, et al. Efficacy of computer-aided detection system and thin-slab maximum intensity projection technique in the detection of pulmonary nodules in patients with resected metastases. Invest Radiol 2009;44:105-13. [Crossref] [PubMed]
- Kang MC, Kang CH, Lee HJ, et al. Accuracy of 16-channel multi-detector row chest computed tomography with thin sections in the detection of metastatic pulmonary nodules. Eur J Cardiothorac Surg 2008;33:473-9. [Crossref] [PubMed]
- Chung CC, Hsieh CC, Lee HC, et al. Accuracy of helical computed tomography in the detection of pulmonary colorectal metastases. J Thorac Cardiovasc Surg 2011;141:1207-12. [Crossref] [PubMed]
- Marron MC, Lora D, Gamez P, et al. Agreement Between Computed Tomography and Pathologic Nodule Counts in Colorectal Lung Metastases. Ann Thorac Surg 2016;101:259-65. [Crossref] [PubMed]
- Eckardt J, Licht PB. Thoracoscopic or open surgery for pulmonary metastasectomy: an observer blinded study. Ann Thorac Surg 2014;98:466-9; discussion 469-70. [Crossref] [PubMed]
- Lin MW, Chen JS. Image-guided techniques for localizing pulmonary nodules in thoracoscopic surgery. J Thorac Dis 2016;8:S749-55. [Crossref] [PubMed]
- Murakawa T, Sato H, Okumura S, et al. Thoracoscopic surgery versus open surgery for lung metastases of colorectal cancer: a multi-institutional retrospective analysis using propensity score adjustment†. Eur J Cardiothorac Surg 2017;51:1157-63. [Crossref] [PubMed]
- Meng D, Fu L, Wang L, et al. Video-assisted thoracoscopic surgery versus open thoracotomy in pulmonary metastasectomy: a meta-analysis of observational studies. Interact Cardiovasc Thorac Surg 2016;22:200-6. [Crossref] [PubMed]
- Welter S, Arfanis E, Christoph D, et al. Growth patterns of pulmonary metastases: Should we adjust resection techniques to primary histology and size? Eur J Cardiothorac Surg 2017;52:39-46. [Crossref] [PubMed]
- Shiono S, Okumura T, Boku N, et al. Outcomes of segmentectomy and wedge resection for pulmonary metastases from colorectal cancer. Eur J Cardiothorac Surg 2017;51:504-10. [PubMed]
- Molins L, Hernandez J, Fibla JJ, et al. Anatomical Resection Improves Survival Over Wedge Resection of Pulmonary Metastases of Colorectal Origin in the Spanish Prospective Multicenter Study (GECMP-CCR). Ann Oncol 2015;26:i45-i47. [Crossref]
- Renaud S, Seitlinger J, Al Lawati Y, et al. Anatomical resections improve survival following lung metastasectomy of colorectal cancer harboring KRAS mutations. Ann Surg 2019;270:1170-7. [Crossref] [PubMed]
- Chung JH, Lee SH, Yi E, et al. Impact of resection margin length and tumor depth on the local recurrence after thoracoscopic pulmonary wedge resection of a single colorectal metastasis. J Thorac Dis 2019;11:1879-87. [Crossref] [PubMed]
- Sun F, Chen L, Shi M, et al. Prognosis of video-assisted thoracoscopic pulmonary metastasectomy in patients with colorectal cancer lung metastases: an analysis of 154 cases. Int J Colorectal Dis 2017;32:897-905. [Crossref] [PubMed]
- Detterbeck FC, Grodzki T, Gleeson F, et al. Imaging requirements in the practice of pulmonary metastasectomy. J Thorac Oncol 2010;5:S134-9. [Crossref] [PubMed]
- Brandman S, Ko JP. Pulmonary nodule detection, characterization, and management with multidetector computed tomography. J Thorac Imaging 2011;26:90-105. [Crossref] [PubMed]
- Perentes JY, Krueger T, Lovis A, et al. Thoracoscopic resection of pulmonary metastasis: Current practice and results. Crit Rev Oncol Hematol 2015;95:105-13. [Crossref] [PubMed]
- Molnar TF, Gebitekin C, Turna A. What are the considerations in the surgical approach in pulmonary metastasectomy? J Thorac Oncol 2010;5:S140-4. [Crossref] [PubMed]
- Althagafi KT, Alashgar OA, Almaghrabi HS, et al. Missed pulmonary metastasis. Asian Cardiovasc Thorac Ann 2014;22:183-6. [Crossref] [PubMed]
- Welter S, Barile La Raia R, Gupta V. Pursuit of an optimal surgical margin in pulmonary metastasectomy. J Vis Surg 2019;5:39. [Crossref]
Cite this article as: Welter S, Gupta V. Algorithm for the pulmonary metastasectomy based on number of metastases and histology. Video-assist Thorac Surg 2021;6:35.


