
Oncological clearance of minimally invasive lobectomy for clinical N0 non-small cell lung cancer: the role of robotic surgery
IntroductionOther Section
- Introduction
- Long-term survival of early-stage NSCLC following robotic lobectomy
- Nodal upstaging as an indicator of oncological efficacy of robotic lobectomy
- Conclusions
- Acknowledgments
- Footnote
- References
In recent years, minimally invasive surgery became the gold standard for the radical treatment of early-stage non-small cell lung cancer (NSCLC) (1). In fact, several studies demonstrated better perioperative outcomes compared to open thoracotomic surgery, in particular in terms of postoperative pain, incidence of major and minor complications, chest tube duration, functional recovery, postoperative hospitalization, and readmission rate (1).
Several authors demonstrated the feasibility of robotic-assisted lobectomy as an alternative to video-assisted thoracic surgery (VATS) for early-stage NSCLC treatment (2-4). The robotic platform allows improved vision and better maneuverability, which have been widely recognized as points of strength compared to VATS technique. In 2010, our group proposed a modified four-arm approach, the robotic-assisted lobectomy, including three 8-mm robotic ports and a 2.5-cm utility incision at the fourth intercostal space for the introduction of the fourth robotic arm and accessory instruments by the table assistant (2). Dylewski and Cerfolio later described a closed-chest complete portal approach with carbon dioxide insufflation (3,4), in which, as in open lobectomy, hilar structures are approached in a posterior-to-anterior direction. In the robotic-assisted lobectomy, instead, we adopt a “fissureless” technique, with isolation of vascular and bronchial elements proceeding from the anterior mediastinum (2).
Despite the limited diffusion of the system because of initial costs, the proportion of patients undergoing robotic lobectomy progressively raised up to about 15% overall (5). The analysis of immediate outcomes showed non-inferior results compared to VATS and open surgery after completion of the learning curve in several studies (6). In a propensity-matched study, the robotic approach showed lower complication and mortality rates and earlier discharge compared to open thoracotomy (7). From the analysis of STS database, no differences other than longer surgical time were evident in terms of intraoperative bleeding, ICU admission, need of reoperation, postoperative complications, and hospital stay in VATS vs. robotic lobectomy (5). These results were further confirmed by a recent meta-analysis by the Shanghai Pulmonary Hospital group; moreover, 30-day mortality in patients treated with robotics resulted significantly lower than VATS patients (8).
While the number of studies reporting results of perioperative outcomes of robotic lobectomy is constantly growing, still limited data are available on the oncological radicality of the technique in patients undergoing treatment of cN0 disease. The aim of this study is to review the results of the currently existing literature reporting data on long-term survival in patients with early-stage NSCLC who underwent robotic lobectomy. Moreover, since the rate of intraoperative nodal upstaging has been identified as a surrogate of oncological effectiveness, we examined the studies that compared this outcome between robotically treated patients and those who underwent VATS or open surgery.
Long-term survival of early-stage NSCLC following robotic lobectomyOther Section
- Introduction
- Long-term survival of early-stage NSCLC following robotic lobectomy
- Nodal upstaging as an indicator of oncological efficacy of robotic lobectomy
- Conclusions
- Acknowledgments
- Footnote
- References
Similarly to what happened after the introduction of VATS lobectomy, the value of robotic lobectomy in terms of oncological radicality became a matter of debate following the publication of the first clinical series (2-4). Despite medium- and long-term survival data of single-center studies were encouraging, evidences based on multi-center data are still limited. The main clinical characteristics, perioperative data, and survival results of studies on the robotic treatment of cN0 NSCLC patients are enlisted in Table 1. These studies report survival data exceeding 5 years after surgery in a considerable number of the patients enrolled. However, the overall short median follow-up, ranging between 24 and 45 months, has still to be considered a major limitation in the evaluation of long-term results in this cohort of patients.
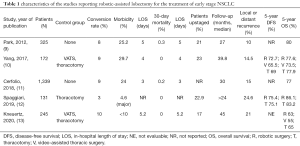
Full table
In 2012, the study by Park et al. reported perioperative and long-term survival results of 325 patients affected by early-stage NSCLC treated by robotic lobectomy in three large-volume centers (9). Most patients (95%) had cN0 disease. Among the entire cohort, in 24% of cases the final pathologic analysis revealed a more advanced disease stage (II–IIIA). The patients were followed up postoperatively for a median of 27 months, and the overall survival (OS) 5 years after surgery was 80%. Tumor recurred in 10% of patients, most of them with distant metastases. The authors concluded that survival after robotic lobectomy for early-stage NSCLC gained excellent results comparable to data previously reported for patients treated by means of VATS or open thoracotomy. However, the lack of a control arm (either VATS or thoracotomic) was identified as a major limitation of the study.
This experience was updated in 2018 with the enrollment of 1,339 patients overall from four independent institutions (10). A number of patients with locally advanced or with separate metastatic pulmonary nodules were also included in the analysis. In 91 patients, unforeseen N2 metastatic lymph nodes were identified at pathologic staging. Even if the median follow-up increased by only 3 months compared to the study released in 2012, the percentage of patients evaluated up to 5 years after the operation raised to about a third of the entire cohort. A slight decrease of 5-year OS was observed (77%). The reduced inflammatory status induced by minimally invasive robotic approach and enhanced lymph node dissection were identified as points of strength to achieve longer survival compared to open approach.
Three additional single-center trials investigated the results of robotic lobectomy for the treatment of early-stage NSCLC. Perioperative and long-term survival data were analyzed after matching patients with comparable control groups of VATS and open surgery cases in two studies (11,12), and open surgery alone in one further study (13). In one trial, a small population (6%) of patients with II or IIIA stage disease was collectively analyzed with early-stage cases (12).
Robotic lobectomy resulted uniformly to be a safe technique. In fact, no case of in-hospital perioperative deaths and low rate of major complications were reported by authors, with no significant difference when compared to control groups. The need of thoracotomic conversion ranged around 9% (11-13).
In about 20% of patients, the final pathology report showed a migration of clinical I stage toward a more advanced disease. In particular, the discovery of metastatic N1 and/or N2 lymph nodes resulted the main determinant for disease upstaging (17% of patients in the study of Kneuertz et al.) (12). Nevertheless, 10% to 24% of patients developed later recurrence of disease at follow-up investigations. Relapses occurred mostly on distant sites (12).
Long-term OS 5 years after surgical intervention was consistent with previous reports (77.6%) in the study of Yang et al. (11). While Spaggiari and colleagues reported excellent survival overcoming 86% (13), survival rate dropped to 63% in the trial of Kneuertz et al. A longer follow-up compared to other studies and the inclusion of clinical and pathological stage II and III NSCLC patients could be responsible for worse survival (12). In all these studies, survival outcomes of robotic lobectomy were comparable with no significant statistical difference to those obtained by VATS and open thoracotomic lobectomy.
Nodal upstaging as an indicator of oncological efficacy of robotic lobectomyOther Section
- Introduction
- Long-term survival of early-stage NSCLC following robotic lobectomy
- Nodal upstaging as an indicator of oncological efficacy of robotic lobectomy
- Conclusions
- Acknowledgments
- Footnote
- References
In oncological surgery, survival is strongly dependent on the stage of disease at the time of diagnosis and treatment. The implementation of pathologic staging increases the potential identification of patients affected by minimal burden of metastatic disease. The so-called “Will Rogers phenomenon” emphasizes this concept (14): with the improvement of the adequacy of staging techniques, patients with micro-metastases migrate to higher stages of disease, and survival rate consequently increases in both early and advanced disease groups of patients.
According to the Tumor-Node-Metastasis (TNM) staging system, the presence of nodal involvement is the most important descriptor affecting survival. In a meta-analysis, the pooled negative predictive value (NPV) of combined positron emission tomography (PET) and computed tomography (CT) scan staging resulted 93% for the identification of mediastinal metastases in patients affected by T1–T2 NSCLC (15). Yet, given the high NPV of non-invasive staging, intraoperative diagnosis of occult lymph node metastases and disease upstaging are indirect indicators of oncological efficacy of surgical techniques, because adequate adjuvant therapies can be consequently delivered to properly staged patients.
A few studies analyzed the performance of robotic lymph node dissection in early-stage NSCLC patients in terms of nodal upstaging, and compared the results with those obtained by VATS and open surgery. There is a large agreement that the robotic system allows better dissection of lymphatic structures even in presence of fibrotic tissues, better control of hemostasis and lymphatic leakage, especially compared to VATS surgery (Figure 1) (11).
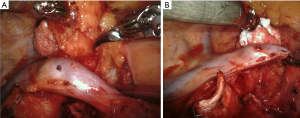
Wilson and colleagues reported the results of a multi-center analysis on 302 patients with clinical I stage NSCLC treated by robotic lobectomy or segmentectomy (16). In 10.9% of cases, pathologic upstaging was observed due to the presence of pN1 or pN2 metastases. An upstaging rate increase was observed according to clinical T parameter. When compared to historical studies, robotic upstaging denoted better performance than VATS and similar results to open thoracotomic lobectomy.
A few other studies investigated the prevalence of nodal upstaging in patients undergoing robotic lobectomy for cN0 NSCLC (17-21), ranging from about 10% to 20% overall. Zirafa et al. compared two groups of 106 patients treated by either robotic or thoracotomic lobectomy (17). Intraoperative nodal upstaging resulted higher, however not statistically different, in the robotic group than in the open surgery group (20.8% vs. 17.9%, respectively). Nevertheless, robotic surgery allowed a higher cN0 to pN2 upstaging (9.4% vs. 2.8%, P=0.04), resulting from enhanced 3-dimensional magnified robotic visualization of mediastinal compartment, according to the authors.
A recent analysis from the U.S. National Cancer Database (NCDB) conducted by Tang et al. confirmed similar rate of nodal upstaging in over 7,000 matched pairs of patients treated for stage I NSCLC by robotic or open lobectomy (11% vs. 11.6%, P=0.28) (18). A significantly higher number of lymph nodes were harvested in the robotic cases. Despite perioperative mortality resulted lower in the robotic group, similar 5-year OS was obtained by both techniques (65.6% in the robotic group vs. 66.7% in the thoracotomy group, P=0.25).
Therefore, robotic surgery allows a nodal upstaging rate similar to open thoracotomic surgery. Yet, contrasting results have been reported in the literature when compared to VATS (19-21). In the single-center study by Lee and colleagues (19), equivalent nodal upstaging was evident in 13.2% of 53 patients treated by robotic lobectomy compared to 15.2% obtained in the VATS lobectomy group (158 patients, P=0.72). The subgroup analysis according to T parameter confirmed no difference related to surgical technique.
In a propensity-matched study analyzing the performance of robotic, videothoracoscopic, and open surgery lobectomy (20), pathologic nodal upstaging resulted significantly higher in patients treated by robotic technique than VATS, but lower than that of the thoracotomic cohort (16.2% vs. 12.3% vs. 21.8%, respectively; P=0.03). Moreover, difference between robotic and open surgical groups was not furtherly confirmed at multivariate analysis.
Superiority of robotic nodal upstaging to VATS was not confirmed by a recent study by Hennon et al. (21). Outcome data of clinical I stage NSCLC patients who underwent pulmonary lobectomy by robotic, VATS or thoracotomy were extracted from the U.S. NCDB database, and propensity matched. In this trial, nodal upstaging obtained by robotic surgery was close but lower compared to VATS and open surgery (10.8% vs. 11.1% vs. 12.1%, respectively; P<0.01).
Okusanya and colleagues (22) identified a potential factor influencing these mixed results. The authors analyzed the prevalence of nodal upstaging after robotic lobectomy comparing the results according to hospital volume. A significant higher proportion of patients was shown to have a higher number of lymph nodes harvested as well as occult lymph node metastases at intraoperative evaluation in large-volume centers (i.e., those performing over 53 robotic lobectomies per year) than others (P<0.001). Therefore, an improvement of nodal upstaging results can be expected accordingly with the growth of experience with robotic lobectomy. However, additional studies are needed for a definitive evaluation of the outcome of nodal staging of robotic surgery, in particular compared to VATS lobectomy.
ConclusionsOther Section
- Introduction
- Long-term survival of early-stage NSCLC following robotic lobectomy
- Nodal upstaging as an indicator of oncological efficacy of robotic lobectomy
- Conclusions
- Acknowledgments
- Footnote
- References
The long-term survival in patients undergoing robotic lobectomy for cN0 NSCLC was shown to be equivalent to that reported by VATS lobectomy and open thoracotomic surgery studies. However, these data are still supported by a limited number of multi-center trials with short median follow-up. Further large-volume prospective studies with extended follow-up are advocated to definitely assess the oncological benefits of robotic lobectomy on long-term survival compared to other approaches in the treatment of early-stage lung cancer.
AcknowledgmentsOther Section
- Introduction
- Long-term survival of early-stage NSCLC following robotic lobectomy
- Nodal upstaging as an indicator of oncological efficacy of robotic lobectomy
- Conclusions
- Acknowledgments
- Footnote
- References
The authors thank Dr. Francesca Rossetti for language review.
Funding: None.
FootnoteOther Section
- Introduction
- Long-term survival of early-stage NSCLC following robotic lobectomy
- Nodal upstaging as an indicator of oncological efficacy of robotic lobectomy
- Conclusions
- Acknowledgments
- Footnote
- References
Provenance and Peer Review: This article was commissioned by the Guest Editor (Mingyon Mun) for the series “Oncological clearance of VATS lobectomy for clinical N0 non-small cell lung cancer” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats-20-42). The series “Oncological clearance of VATS lobectomy for clinical N0 non-small cell lung cancer” was commissioned by the editorial office without any funding or sponsorship. GV reports grants from INAIL, during the conduct of the study; personal fees from Medtronic, personal fees from Ab Medica, personal fees from Johnson & Johnson, grants from Intuitive, outside the submitted work. The other author has no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Introduction
- Long-term survival of early-stage NSCLC following robotic lobectomy
- Nodal upstaging as an indicator of oncological efficacy of robotic lobectomy
- Conclusions
- Acknowledgments
- Footnote
- References
- Ikeda N. Updates on Minimally Invasive Surgery in Non-Small Cell Lung Cancer. Curr Treat Options Oncol 2019;20:16. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg 2011;23:36-42. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Louie BE, Wilson JL, Kim S, et al. Comparison of Video-Assisted Thoracoscopic Surgery and Robotic Approaches for Clinical Stage I and Stage II Non-Small Cell Lung Cancer Using The Society of Thoracic Surgeons Database. Ann Thorac Surg 2016;102:917-24. [Crossref] [PubMed]
- Veronesi G. Robotic thoracic surgery: technical considerations and learning curve for pulmonary resection. Thorac Surg Clin 2014;24:135-41. v. [Crossref] [PubMed]
- Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-Assisted, Video-Assisted Thoracoscopic and Open Lobectomy: Propensity-Matched Analysis of Recent Premier Data. Ann Thorac Surg 2017;104:1733-40. [Crossref] [PubMed]
- Hu J, Chen Y, Dai J, et al. Perioperative outcomes of robot-assisted vs video-assisted and traditional open thoracic surgery for lung cancer: A systematic review and network meta-analysis. Int J Med Robot 2020;16:1-14. [Crossref] [PubMed]
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. [Crossref] [PubMed]
- Cerfolio RJ, Ghanim AF, Dylewski M, et al. The long-term survival of robotic lobectomy for non-small cell lung cancer: A multi-institutional study. J Thorac Cardiovasc Surg 2018;155:778-86. [Crossref] [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-term Survival Based on the Surgical Approach to Lobectomy For Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg 2017;265:431-7. [Crossref] [PubMed]
- Kneuertz PJ, D'Souza DM, Richardson M, et al. Long-Term Oncologic Outcomes After Robotic Lobectomy for Early-stage Non-Small-cell Lung Cancer Versus Video-assisted Thoracoscopic and Open Thoracotomy Approach. Clin Lung Cancer 2020;21:214-24.e2. [Crossref] [PubMed]
- Spaggiari L, Sedda G, Maisonneuve P, et al. A Brief Report on Survival After Robotic Lobectomy for Early-Stage Lung Cancer. J Thorac Oncol 2019;14:2176-80. [Crossref] [PubMed]
- Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med 1985;312:1604-8. [Crossref] [PubMed]
- Wang J, Welch K, Wang L, et al. Negative predictive value of positron emission tomography and computed tomography for stage T1-2N0 non-small-cell lung cancer: a meta-analysis. Clin Lung Cancer 2012;13:81-9. [Crossref] [PubMed]
- Wilson JL, Louie BE, Cerfolio RJ, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg 2014;97:1901-6; discussion 1906-7. [Crossref] [PubMed]
- Zirafa C, Aprile V, Ricciardi S, et al. Nodal upstaging evaluation in NSCLC patients treated by robotic lobectomy. Surg Endosc 2019;33:153-8. [Crossref] [PubMed]
- Tang A, Raja S, Bribriesco AC, et al. Robotic Approach Offers Similar Nodal Upstaging to Open Lobectomy for Clinical Stage I Non-small Cell Lung Cancer. Ann Thorac Surg 2020;110:424-33. [Crossref] [PubMed]
- Lee BE, Shapiro M, Rutledge JR, et al. Nodal Upstaging in Robotic and Video Assisted Thoracic Surgery Lobectomy for Clinical N0 Lung Cancer. Ann Thorac Surg 2015;100:229-33; discussion 233-4. [Crossref] [PubMed]
- Kneuertz PJ, Cheufou DH, D'Souza DM, et al. Propensity-score adjusted comparison of pathologic nodal upstaging by robotic, video-assisted thoracoscopic, and open lobectomy for non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:1457-1466.e2. [Crossref] [PubMed]
- Hennon MW, DeGraaff LH, Groman A, et al. The association of nodal upstaging with surgical approach and its impact on long-term survival after resection of non-small-cell lung cancer. Eur J Cardiothorac Surg 2020;57:888-95. [Crossref] [PubMed]
- Okusanya OT, Lutfi W, Baker N, et al. The association of robotic lobectomy volume and nodal upstaging in non-small cell lung cancer. J Robot Surg 2020;14:709-15. [Crossref] [PubMed]
Cite this article as: Muriana P, Veronesi G. Oncological clearance of minimally invasive lobectomy for clinical N0 non-small cell lung cancer: the role of robotic surgery. Video-assist Thorac Surg 2021;6:15.


