
Prognostic factors and recurrence dynamics after multiple-port video-assisted thoracoscopic lobectomy for clinical T1-3N0 non-small cell lung cancer
Introduction
Over the past three decades, video-assisted thoracoscopic surgery (VATS) has emerged as a preferred surgical approach when performing lobectomy for patients with early-stage non-small cell lung cancer (NSCLC). Owing to the initial efforts of pioneers in this minimally invasive approach (1-7), thoracic surgeons have been increasingly adopting VATS lobectomy (8,9). This led to the accumulation of clinical evidence based on single- and multi-institutional retrospective studies, reporting that early and late outcomes of VATS were comparable to or even superior to those of open thoracotomy (1-17). Recently, several large-scale analyses from national databases have demonstrated that VATS lobectomy can be performed with improved short-term outcomes (18-25) and with favorable long-term survival compared with thoracotomy lobectomy (26). Therefore, VATS lobectomy is now strongly recommended for patients with no medical and surgical contraindications, as long as oncologic principles of surgery is not compromised.
However, questions still remain about whether VATS lobectomy is oncologically effective (14). Most previous studies demonstrating that VATS was not inferior to thoracotomy in terms of survival outcomes might have been subject to selection bias (12). Additionally, given the conflicting results on the incidence of pathologic nodal upstaging after VATS, there are concerns over the adequacy of intraoperative lymph node (LN) assessment during VATS lobectomy (15,24,26-29). Increasing interests in single-port VATS lobectomy might reinforce doubt on the quality of LN assessment through minimally invasive approach (30-33).
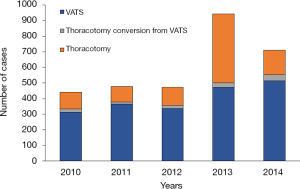
As an early adopter, since we reported our initial experience (13), our institution has been continuously raising the proportion of VATS among curative-intent surgical cases (Figure 1). It is worthwhile to contribute a high-volume institution’s experience on multi-port VATS lobectomy to the current literature on oncological efficacy of VATS lobectomy. Therefore, the objectives of this study were to report our institutional surgical outcomes, including early postoperative course, pathologic nodal upstaging, and long-term survival in patients who underwent VATS lobectomy for clinical T1-3N0M0 NSCLC and to analyze the prognostic factors and recurrence dynamics in this cohort.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/vats-19-58).
Methods
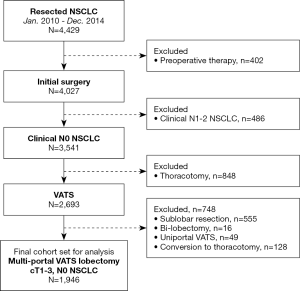
Between January 2010 and December 2014, a total of 4,429 consecutive patients underwent curative-intent surgery for NSCLC at our institution. Among these, 2,074 patients who underwent VATS lobectomy for clinical T1-3N0M0 disease were recognized (Figure 2). VATS approach was initially attempted in these patients and of those, we had to convert to open thoracotomy in 128 patients (6.2%). Eventually, the remaining 1,946 patients were focused on this study and their medical records were retrospectively reviewed to assess clinicopathologic characteristics, early postoperative outcomes, recurrence pattern, and survival. The study design of this research is retrospective cohort study and is not related to an experimental study (interventional study) of human being. Therefore, we did not include the Declaration of Helsinki in the manuscript. This study was reviewed and approved by the Institutional Review Board of Samsung Medical Center (IRB No. 2020-08-056). Individual consent for this retrospective analysis was waived.
The routine preoperative workup included a complete history and physical examination, complete blood counts, chemistry profiles, pulmonary function tests (PFTs), simple chest X-ray, computed tomography (CT) of the chest and upper abdomen, bronchoscopy with washing cytology and/or biopsy, integrated whole-body 18F-fluorodeoxyglucose (FDG) positron emission tomography and CT (PET/CT) scans, and brain magnetic resonance imaging (MRI). For patients with suspicious nodal involvement, histopathologic examination through endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) were conducted to rule out nodal metastasis. Candidates for VATS lobectomy were patients with clinical T1-3N0M0 disease, peripherally located lesions (no endobronchial lesions), and a tumor of 6 cm in diameter or smaller. Patients were required to be able to tolerate single-lung ventilation, as determined by preoperative PFTs.
In general, VATS lobectomy was performed under single-lung anesthesia, using two ports and a utility incision without rib spreading. A 15 mm trocar for the 10 mm, 30-degree thoracoscope was placed through the seventh or eighth intercostal space in the posterior axillary line. A 4 cm utility incision was made through the fourth or fifth intercostal space in the anterior axillary line. An additional 5 mm trocar was placed through the sixth or seventh intercostal space in the posterior scapular line. However, details in surgical techniques of VATS lobectomy varied among surgeons as each surgeon has modified them according to their preferences. Despite these technical variances, oncological principles should be always complied with as follows: (I) the vessels and bronchi of the target lobe were individually dissected, (II) systematic LN dissection was regarded as mandatory, and (III) touching the LN itself and rupturing the capsule of the LN was avoided. Mediastinal LN dissection consisted of en bloc resections of all nodes at stations 2R, 4R, 7, 8, 9, and 10R for right-sided tumors and nodes at stations 4L, 5, 6, 7, 8, 9 and 10L for left-sided tumors. When LN enlargement was observed or LN metastasis was suspected, frozen section biopsies were performed during surgery. All specimens were placed into an impermeable bag and removed through the utility incision.
For patients with pathologic stage II or more advanced disease, adjuvant chemotherapy, radiotherapy or chemoradiation was administered as long as they were able to tolerate additional treatments. Patients were regularly evaluated by CT every 3 to 4 months for the first 2 years following surgery, and then every 6 months thereafter. Patients were evaluated by PET/CT scans when recurrence was suspected. Loco-regional recurrence was defined as that occurring within the ipsilateral hemithorax, including the pleura and mediastinal LNs. Distant recurrence was defined as that developing within the contralateral hemithorax or a distant solid organ. Whenever recurrence was suspected, we tried to obtain histological or unequivocal radiological proof. In cases lost to follow-up, a telephone interview was conducted to determine late outcomes.
Descriptive statistics were used to assess patient demographic characteristics and outcomes. Normally distributed continuous data were expressed as means ± standard deviations (SDs) and were compared using Student’s t-test. Non-normally distributed continuous data were expressed as median (interquartile range) and were compared using Mann-Whitney U test. Categorical data were expressed as counts and proportions. Student’s t-tests and the χ2 test or Fisher’s exact test were used to compare continuous and categorical variables, respectively. Overall survival was defined as the time from the date of surgery until the last date of follow-up for patients who remained alive or until death. Recurrence-free survival was defined as the time from the date of surgery to the confirmed date of non-evidence of recurrence or to the date of recurrence. Survival curves were estimated using the Kaplan-Meier method and were compared using the log-rank test. Monthly hazard rates were estimated at 3-month intervals using a kernel-Epanechnikov smoothing method. The optimal cutoff value of harvested LN for predicting unexpected pN1 or pN2 was estimated using receiver operating characteristics curve analysis. The optimal cutoff count of harvested LNs for predicting overall survival and recurrence-free survival were estimated using maximally selected rank statistics with the R package maxstat and survminer (0.4.4). To determine which factors were associated with unexpected N1 or N2 diseases, a multivariable logistic regression model evaluating age, sex, body mass index (BMI), smoking history, forced expiratory volume in 1 second (FEV1), diffuse capacity of carbon monoxide, Charlson comorbidity index, anatomic location of the tumor, tumor size, clinical T category, histology, number of harvested LNs, optimal cutoff of harvested LNs, and dissected number of N2 stations were performed; nonsignificant variables were excluded in a stepwise fashion to obtain the final model. To determine which factors were significantly associated with survival, a multivariable analysis using Cox proportional hazards model was performed. All statistical tests were two-sided with a significance level set at 0.05 and were performed using R Studio (version 1.138) utilizing R statistical language version 3.4.2.
Results
Clinical and pathologic features
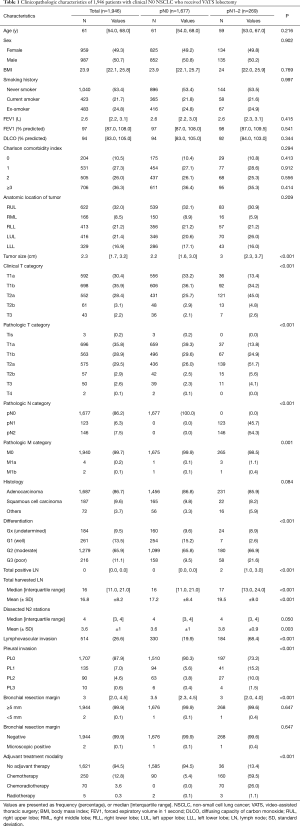
The study included 987 men and 959 women with a mean age of 61 (interquartile range, 54 to 68) years. Details on clinicopathologic features of the study cohort are summarized in Table 1. The most common histologic type was adenocarcinoma (1,687 patients, 87%). Preoperative staging workup including EBUS-TBNA revealed that all patients had clinical N0 disease. During surgery, LN metastases were confirmed by frozen section biopsy in 43 patients (2.2%), but none of these patients underwent conversion to open thoracotomy. The mean number of harvested LNs was 16 (interquartile range, 11 to 21). Except for two cases of microscopic residual tumor at the bronchial resection margin, there were no cases of incomplete resection.
Full table
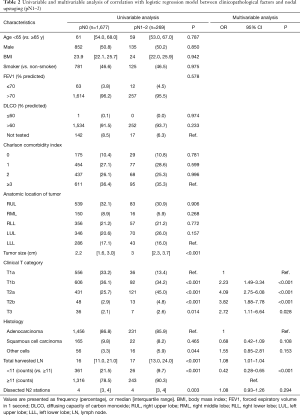
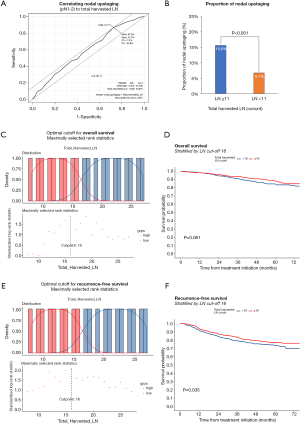
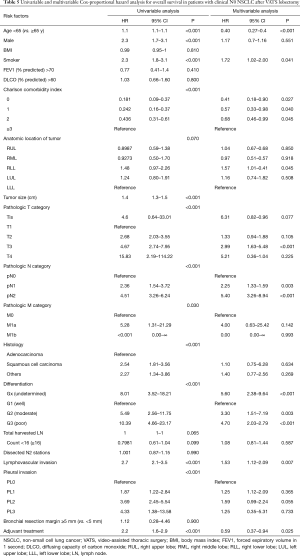
Pathologic N1 and N2 diseases were finally confirmed in 123 (6.3%) and 146 patients (7.5%), respectively. The optimal cut-off counts of harvested LNs for predicting nodal upstaging calculated by receiver operating characteristic curve was 11 (sensitivity 87.0%, specificity 27.5%). On a multivariable logistic regression analysis, higher clinical T category and 11 or more total harvested LNs were independent predictors for detecting unexpected pathologic N1 or N2 diseases (Table 2). In patients with pathologic N2 disease, 120 patients (82.2%) had single-station involvement and 67 patients (45.9%) had extracapsular invasion. The median diameter of the largest metastatic LNs was 4 (interquartile range, 3 to 8) mm.
Full table
Early postoperative outcomes
Two in-hospital mortalities (0.1%) and 441 complications (22.7%) occurred during the early postoperative period. The most common complication was prolonged air leak (109 patients, 5.6%). The median length of hospital stay was 6 (interquartile range, 5 to 7) days. Details of early postoperative outcomes are listed in Table 3. Postoperatively, 322 patients (16.7%) underwent adjuvant chemotherapy (n=247), chemoradiation (n=70), or radiotherapy (n=5). Among the remaining 1,621 patients (94.5%), 62 patients (3.2%) were candidates for adjuvant therapy, but they did not undergo adjuvant treatment due to following reasons: Old age or poor performance status (n=25), patient refusal (n=18), comorbidity (n=7), disease progression (n=7), loss of follow-up (n=6), and no social support (n=1). The median time interval between the discharge from surgery and the start of adjuvant treatment was 34 (interquartile range, 28 to 40) days. Of the 247 patients who received chemotherapy, 197 (79.8%) received their full planned dose on schedule without delay. Eighteen patients (7.3%) completed planned schedule, but their regimen was changed during the courses (dose reduction in 13, regimen change in 5) with delay in 1 or more cycles. The remaining 32 patients (12.9%) stopped adjuvant treatment during the courses due to toxicities. Reasons for dose reduction or regimen change were peripheral neuropathy (n=7), neutropenia (n=5), gastrointestinal symptoms (n=3), and unknown cause (n=2).
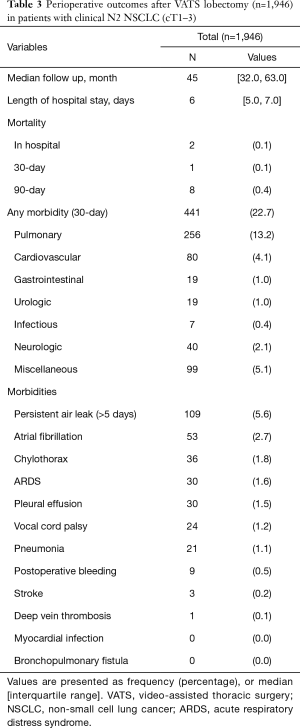
Full table
Survival and recurrence pattern
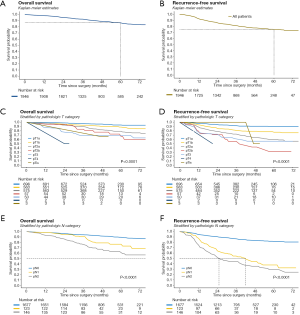
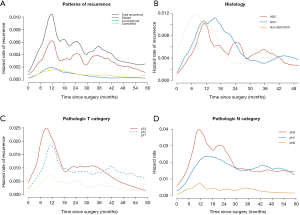
Follow-up was complete for all patients. The median follow-up time was 45 months. At the end of follow-up, there were 1,728 surviving patients (88.8%). Overall survival at 5 years were 89.6% in pathologic N0, 76.5% in pathologic N1, and 61% in pathologic N2 (Figure 3). During follow-up, 364 patients (18.7%) developed recurrence. The pattern of recurrence was loco-regional in 67 patients, distant in 226, and both in 71 (Table 4). Recurrence dynamics analysis showed that most recurrences occurred within the initial 2 years of postoperative follow-up. The peak incidence of overall recurrence as well as distant metastasis was detected twice (approximately 12 and 24 months after the operation), whereas that of local recurrence was detected one (approximately 12 months after the operation) (Figure 4). Recurrence-free survival at 5 years were 81.7% in pathologic N0, 37.8% in pathologic N1, and 29.2% in pathologic N2 (Figure 3).
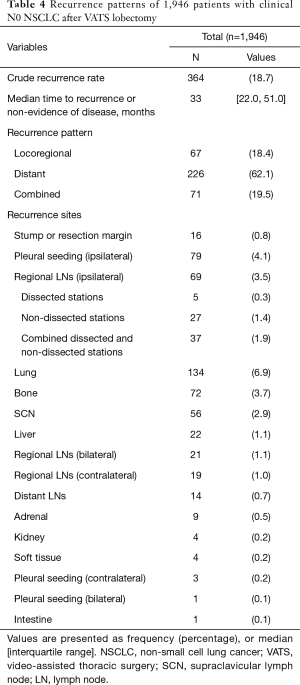
Full table
Prognostic factor analysis
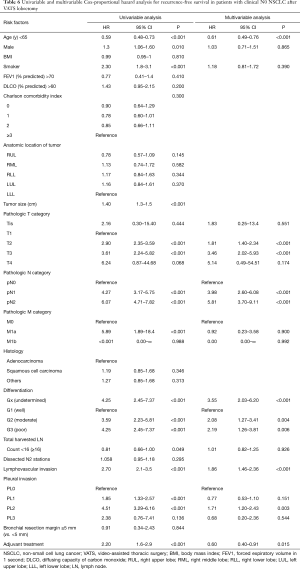
Optimal cut-off counts of total harvested LNs for survival prediction were 16 for both overall survival and recurrence-free survival (Figure 5). To better understand the factors associated with improved outcomes, univariable and multivariable analyses were performed and are detailed in Tables 5,6. Age (≥65 years), smoking history, higher Charlson comorbidity index, pathologic T category, pathologic N category, tumor differentiation, lymphovascular invasion were significantly associated with both worse overall survival and recurrence-free survival. However, adjuvant therapy was significantly associated with favorable overall survival (HR, 0.59; 95% CI, 0.73–0.94; P=0.025) and recurrence-free survival (HR, 0.60; 95% CI, 0.40–0.91; P=0.015).
Full table
Full table
Discussion
Previous institutional studies have demonstrated that VATS lobectomy is associated with more favorable short-term results than thoracotomy, including fewer postoperative complication and shorter length of hospital stay (1-17). However, it is doubtful whether the outcomes from such specialized centers can be generalized. Over the past 5 years, several large-scale studies using a national database have been published to resolve the doubts. They demonstrated the short-term safety and feasibility of VATS lobectomy even in real-world practice (18-25). Despite these favorable short-term outcomes, VATS lobectomy has not been widely accepted by thoracic surgeons (34), because questions on its oncologic reliability are unsolved (14). These concerns may be partly due to insufficient evidence on its long-term survival. Recently, Yang et al. investigated the long-term survival of VATS versus thoracotomy lobectomy using a national database for the first time. They found that VATS was associated with noninferior long-term survival when compared with thoracotomy lobectomy in real-world data (26). However, since this national study lacks the detailed information such as timing of recurrence or recurrence site, neither disease-free survival nor recurrence pattern analyses are available. In contrast, we have been maintaining strict follow-up plans after curative-intent surgery in our institution. This allowed us to conduct a comprehensive analysis on disease-specific survival as well as recurrence pattern and dynamics. Furthermore, our study cohort consists of homogeneous population who received consistent surgical procedure as well as perioperative treatment protocols. Such advantages of data from a high-volume academic institution cannot be addressed by national database analyses.
Although our findings are based on a single institutional series with a single arm alone, long-term overall survival and recurrence-free survival seems to be very promising when compared with historical controls (35). In particular, it should be noted that our favorable survival outcomes are from patients with clinical N0 disease, which eventually included unexpected pathologic N1 or N2 disease. When confined to pathologic N0 disease regardless of pathologic T stage, the 5-year overall survival rate almost reached 90%, which corresponds to overall survival of pathologic stage IA1 according to the eighth edition proposed by the IASLC Staging and Prognostic Factors Committee (35). Our excellent long-term survival can be explained by the fact that VATS was chosen in highly selected patients. We routinely performed integrated PET/CT scans for accurate staging. For suspicious nodal involvement, EBUS-TBNA was also conducted. VATS lobectomy was planned only when patients had clinical N0 disease based on such meticulous staging workups. This could have made our study cohort enrich patients with truly early-stage NSCLC. This also suggests that even in patients with pathologic nodal involvement unexpectedly detected at surgery, its extent of nodal metastasis is likely to be minimal and microscopic. This is supported by our findings that N2 disease mostly involved single station and that the median diameter of the largest metastatic LNs was only 4 mm.
Another explanation for our improved long-term survival might be that our VATS cohort may have had more patients physiologically fit for postoperative recovery; for instance, younger age, preserved pulmonary function, good performance status, and fewer comorbidities. These features reflect the fact that our patients were highly selected before we decide to choose VATS. Apart from favorable impact of minimally invasive approach on early postoperative recovery, patients with good cardiopulmonary function might have also led to reduced early morbidities and mortalities, in turn resulting in improved overall survival. Many researchers are currently paying attention to the negative effect of postoperative complications on the long-term survival in various types of malignancies (36,37). In the same context, inflammatory markers or acute phase responses might be substantially favorable after VATS in terms of host immune function (38-41). These findings should be validated in further comparative studies involving VATS versus thoracotomy.
Concerns over the oncologic efficacy of VATS also arise from the risk of inadequate LN assessment through a limited access. Several nation-wide analyses showed that VATS was associated with less frequent nodal upstaging when compared with open thoracotomy (24,27,28). However, intrinsic limitations of exposure and manipulation of VATS can be overcome by the highly magnified view from video-assistance and advances in endoscopic instruments. Also, with experiences in VATS lobectomy accumulating, the quality of LN dissection can be improved. Some researchers have shown no significant differences in nodal upstaging between open and VATS (15,26,29). In this study, although we performed VATS in selected patients with clinical N0 disease based on meticulous staging workups, the rate of nodal upstaging was not negligible. Among our 1,946 patients with clinical N0 disease, 269 patients (13.8%) turned out to have pathologic N1 or N2 disease. The median number of harvested LNs was 16, which was not inferior to that of the conventional open approach. In our series, the fact that unexpected N1 or N2 diseases were not uncommon despite thorough meticulous preoperative staging suggests that the quality of intraoperative LN assessment through our VATS techniques was reliable. Above all, survival rates of the pathologically upstaged patients were comparable to those of thoracotomy lobectomy from previous reports, which suggests that opening the chest might not have resulted in more favorable long-term outcomes in this subset of patients.
In this study, despite favorable overall survival, a substantial number of patients experienced recurrence. Thanks to prospectively designed elaborate database and strict follow-up plans of our institution, we were able to analyze the details of recurrence pattern and dynamics. Such a detailed analysis might not be available in studies based on national database or registry. As expected, the most common pattern of recurrence was distant metastasis. Recurrence dynamics analysis showed that most recurrences occurred within the initial 2 years of postoperative follow-up. The peak incidence of overall recurrence as well as distant metastasis was detected twice (approximately 12 and 24 months after the operation), whereas that of local recurrence was detected once (approximately 12 months after the operation). Based on these findings, we should be reminded that patients need to be strictly followed up through a meticulous surveillance for the first 2 years.
Since this is a retrospective study, our findings suffer from potential confounding and selection biases. VATS might have been performed in selected patients with favorable prognostic factors. Furthermore, patients who were converted to thoracotomy during VATS lobectomy were not included in this study. Also, since this is a noncomparative study, we are not able to demonstrate the outcomes of VATS in comparison with thoracotomy. To overcome these limitations, prospective randomized trials should be conducted.
In conclusion, in this retrospective study of large-volume academic center, VATS lobectomy can be performed with excellent short-term and long-term outcomes in clinical stage T1-3N0M0 NSCLC. VATS lobectomy is an oncologically sound procedure in terms of acceptable upstaging, reliable LN assessment, and satisfactory long-term survival. Our findings suggest that for patients staged N0 disease through meticulous preoperative workup, there is no need to convert into conventional thoracotomy even if intraoperative frozen section biopsy of the LNs is positive for malignancy during VATS lobectomy. Further comparative studies are needed and long-terms survival outcome from ongoing randomized controlled trials are awaited.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Mingyon Mun) for the series “Oncological clearance of VATS lobectomy for clinical N0 non-small cell lung cancer” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review..
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/vats-19-58
Data Sharing Statement: Available at http://dx.doi.org/10.21037/vats-19-58
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats-19-58). The series “Oncological clearance of VATS lobectomy for clinical N0 non-small cell lung cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study design of this research is retrospective cohort study and is not related to an experimental study (interventional study) of human being. Therefore, we did not include the Declaration of Helsinki in the manuscript. This study was reviewed and approved by the Institutional Review Board of Samsung Medical Center (IRB No. 2020-08-056). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kirby TJ, Mack MJ, Landreneau RJ, et al. Lobectomy--video-assisted thoracic surgery versus muscle-sparing thoracotomy. A randomized trial. J Thorac Cardiovasc Surg 1995;109:997-1001; discussion 1001-2. [Crossref] [PubMed]
- Sugi K, Kaneda Y, Esato K. Video-assisted thoracoscopic lobectomy achieves a satisfactory long-term prognosis in patients with clinical stage IA lung cancer. World J Surg 2000;24:27-30; discussion 30-1. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [PubMed]
- Shigemura N, Akashi A, Funaki S, et al. Long-term outcomes after a variety of video-assisted thoracoscopic lobectomy approaches for clinical stage IA lung cancer: a multi-institutional study. J Thorac Cardiovasc Surg 2006;132:507-12. [Crossref] [PubMed]
- Mun M, Kohno T. Efficacy of thoracoscopic resection for multifocal bronchioloalveolar carcinoma showing pure ground-glass opacities of 20 mm or less in diameter. J Thorac Cardiovasc Surg 2007;134:877-82. [Crossref] [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Hanna WC, de Valence M, Atenafu EG, et al. Is video-assisted lobectomy for non-small-cell lung cancer oncologically equivalent to open lobectomy? Eur J Cardiothorac Surg 2013;43:1121-5. [Crossref] [PubMed]
- Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;83:1965-70. [Crossref] [PubMed]
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [Crossref] [PubMed]
- Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009;138:419-25. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Kim K, Kim HK, Park JS, et al. Video-assisted thoracic surgery lobectomy: single institutional experience with 704 cases. Ann Thorac Surg 2010;89:S2118-22. [Crossref] [PubMed]
- Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010;139:976-81; discussion 981-3. [Crossref] [PubMed]
- D'Amico TA, Niland J, Mamet R, et al. Efficacy of mediastinal lymph node dissection during lobectomy for lung cancer by thoracoscopy and thoracotomy. Ann Thorac Surg 2011;92:226-31; discussion 231-2. [Crossref] [PubMed]
- Kuritzky AM, Aswad BI, Jones RN, et al. Lobectomy by video-assisted thoracic surgery vs muscle-sparing thoracotomy for stage i lung cancer: a critical evaluation of short- and long-term outcomes. J Am Coll Surg 2015;220:1044-53. [Crossref] [PubMed]
- Mei J, Guo C, Xia L, et al. Long-term survival outcomes of video-assisted thoracic surgery lobectomy for stage I-II non-small cell lung cancer are more favorable than thoracotomy: a propensity score-matched analysis from a high-volume center in China. Transl Lung Cancer Res 2019;8:155-66. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Paul S, Sedrakyan A, Chiu YL, et al. Outcomes after lobectomy using thoracoscopy vs thoracotomy: a comparative effectiveness analysis utilizing the Nationwide Inpatient Sample database. Eur J Cardiothorac Surg 2013;43:813-7. [Crossref] [PubMed]
- Boffa DJ, Dhamija A, Kosinski AS, et al. Fewer complications result from a video-assisted approach to anatomic resection of clinical stage I lung cancer. J Thorac Cardiovasc Surg 2014;148:637-43. [Crossref] [PubMed]
- Paul S, Isaacs AJ, Treasure T, et al. Long term survival with thoracoscopic versus open lobectomy: propensity matched comparative analysis using SEER-Medicare database. BMJ 2014;349:g5575. [Crossref] [PubMed]
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Laursen LØ, Petersen RH, Hansen HJ, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur J Cardiothorac Surg 2016;49:870-5. [Crossref] [PubMed]
- Medbery RL, Gillespie TW, Liu Y, et al. Nodal upstaging is more common with thoracotomy than with VATS during lobectomy for early-stage lung cancer: an analysis from the National Cancer Data Base. J Thorac Oncol 2016;11:222-33. [Crossref] [PubMed]
- Pagès PB, Delpy JP, Orsini B, et al. Propensity score analysis comparing videothoracoscopic lobectomy with thoracotomy: a French Nationwide Study. Ann Thorac Surg 2016;101:1370-8. [Crossref] [PubMed]
- Yang CJ, Kumar A, Klapper JA, et al. A national analysis of long-term survival following thoracoscopic versus open lobectomy for stage I non-small-cell lung cancer. Ann Surg 2019;269:163-71. [Crossref] [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-53; discussion 353. [Crossref] [PubMed]
- Licht PB, Jorgensen OD, Ladegaard L, et al. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg 2013;96:943-9; discussion 949-50. [Crossref] [PubMed]
- Yang CF, Sun Z, Speicher PJ, et al. Use and outcomes of minimally invasive lobectomy for stage I non-small cell lung cancer in the National Cancer Data Base. Ann Thorac Surg 2016;101:1037-42. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Chen D, Du M, Yang T. Uniportal video-assisted thoracoscopic lobectomy for lung cancer. J Thorac Dis 2016;8:1830-3. [Crossref] [PubMed]
- Cheng K, Zheng B, Zhang S, et al. Feasibility and learning curve of uniportal video-assisted thoracoscopic segmentectomy. J Thorac Dis 2016;8:S229-34. [PubMed]
- Sihoe ADL, Gonzalez-Rivas D, Yang TY, et al. High-volume intensive training course: a new paradigm for video-assisted thoracoscopic surgery education. Interact Cardiovasc Thorac Surg 2018;27:365-71. [Crossref] [PubMed]
- Mathisen DJ. Is video-assisted thoracoscopic lobectomy inferior to open lobectomy oncologically? Ann Thorac Surg 2013;96:755-6. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Nathan H, Yin H, Wong SL. Postoperative complications and long-term survival after complex cancer resection. Ann Surg Oncol 2017;24:638-44. [Crossref] [PubMed]
- Nakada T, Noda Y, Kato D, et al. Risk factors and cancer recurrence associated with postoperative complications after thoracoscopic lobectomy for clinical stage I non-small cell lung cancer. Thorac Cancer 2019;10:1945-52. [Crossref] [PubMed]
- Craig SR, Leaver HA, Yap PL, et al. Acute phase responses following minimal access and conventional thoracic surgery. Eur J Cardiothorac Surg 2001;20:455-63. [Crossref] [PubMed]
- Walker WS, Leaver HA. Immunologic and stress responses following video-assisted thoracic surgery and open pulmonary lobectomy in early stage lung cancer. Thorac Surg Clin 2007;17:241-9. ix. [Crossref] [PubMed]
- Li S, Yang Z, Du H, et al. Novel systemic inflammation response index to predict prognosis after thoracoscopic lung cancer surgery: a propensity score-matching study. ANZ J Surg 2019;89:E507-13. [Crossref] [PubMed]
- Li S, Zhang W, Yang Z, et al. Systemic inflammation score as a novel prognostic indicator for patients undergoing video-assisted thoracoscopic surgery lobectomy for non-small-cell lung cancer. J Invest Surg 2019; [Epub ahead of print]. [Crossref] [PubMed]
Cite this article as: Lee J, Shin S, Kim TH, Cho JH, Choi YS, Kim J, Zo JI, Shim YM, Kim HK. Prognostic factors and recurrence dynamics after multiple-port video-assisted thoracoscopic lobectomy for clinical T1-3N0 non-small cell lung cancer. Video-assist Thorac Surg 2020;5:34.


