Awake surgery for lung metastasectomy
Introduction
Surgical metastasectomy is now considered a usual therapeutic strategy for lung metastases (1). Indeed, this operation may substantially prolong survival in some selected patients (2). Moreover, the refining of the diagnostic tools currently available and their increasing use during neoplastic follow up, made possible earlier diagnosis (3). Lung metastases are mostly located in the lung periphery. This anatomical condition makes such lesions easily removable by minimal resection and particularly suitable for video-assisted thoracic surgery (VATS). At present, this technique may be performed through a unique access further reducing the invasiveness of the procedure (4). Ultimately, the introduction of new drugs, the better knowledge physiopathology and the improved confidence in endeavoring thoracic operation with a breathing lung favored the development of programs of awake thoracic surgery (5,6).
As far as we know, the oldest surgical program with this specific aim was created 20 years ago in our institution directed by Professor Tommaso Claudio Mineo (5). Until 2005 patients we used to approach these lesions with a VATS metastasectomy under awake epidural anesthesia and triportal access. More recently, we generally prefer a 3–5 cm uniportal approach with intercostal blockade and sedation (7) (Video 1). Hereby, we present and discuss these different techniques.
Indications
Indications for non-intubated lung metastasectomy must fulfill different requirements, synthetically described in Table 1. The indispensable oncologic requirements entail primary tumor control, ascertained absence of metastases out of the lung and potentially resectable lung oligometastases that means one or two visible lesions for each side. The surgical and namely VATS requirements imply peripheral and small lesion, specifically less than 3 cm at computed tomography able to be removed with a sublobar resection, detectable with instrumental palpation, and low probability of tenacious/diffuse pleuro-pulmonary adherences. Additional VATS requisites are normal coagulation tests, normal body mass index (<30 kg/m2) (4,7). The functional prerequisites need the ability of the patient to tolerate the planned operation, the prediction of adequate postoperative respiratory function and a normal performance status (7). At this point arterial blood gas test, global respiratory function appraisal, echocardiography and cardiologic assessment become necessary. The anesthesiologic conditions for non-intubated surgery require the easy access to airways, permitting in case of necessity to easily convert the anesthesia into intubation mode. A fully informed consent about the risks of awake non-intubated procedure must be always obtained by the patient. Last group of the requirements consists in a balanced and collaborative psychological status. This must be preoperatively assessed by a psychologist with a specific questionnaire: Profile of Mood States (8) and Mini Mental State Examination (9). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.

Full table
Anesthesia
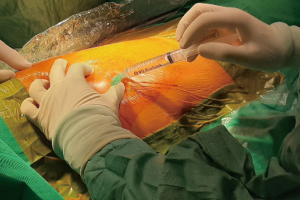
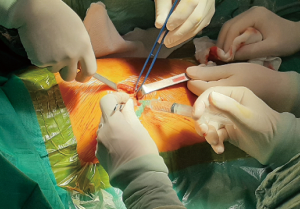
We started our program performing operations with awake and collaborating patients under epidural anesthesia (5,10). Analgesia was performed with thoracic epidural anesthesia, with the catheter placed at T4 level and a solution of ropivacaine 0.5% was continuously infused during the procedure. Thoracic epidural anesthesia may present some unpleasant side-effects, such as hypotension and bradycardia induced by sympathicolysis (10). In addition, the increasing use of anticoagulant therapy in surgical patients may make it difficult to safely perform epidural anesthesia (8). Since 2005 awake thoracic surgery has been conducted with intercostal blockade and with bispectral index monitor to control the level of sedation (11). Intercostal bloc is achieved by lidocaine 2% (4 mg/kg) injected from the incision site (Figure 1) to the parietal pleura followed by the infusion of ropivacaine 7.5% (2 mg/kg) infiltrating the correspondent intercostal nerve (Figure 2). Intrathoracic phases are reinforced with intravenous infusion of benzodiazepine (midazolam 0.03–0.1 mg/kg) or opioids (remifentanil 15 µg/kg/min) or propofol (0.5 mg/kg). These drugs do not interfere with spontaneous breathing (12). To have a good control of the airway during the procedure the anesthesiologist should stay next to the patient. Moreover, a bronchoscope should always be immediately available to rapidly set up for double lumen intubation when necessary. Electrocardiogram, pulse oxymeter, body temperature, systemic and central venous blood pressure, end-tidal CO2 are continuously monitored during the whole operation. It is mandatory timed control of blood gases mostly if the procedures last more than 30 minutes (11).
Surgery
Position
Awake lung metastasectomy is accomplished with the patient in lateral decubitus setting that should be comfortable and long-lasting sustainable. Both arms are moderately elevated and frontally abducted in order to avoid instrumental contrasts. We experienced that the most convenient position for the main operator is ventrally to the patient with the assistant standing aside and caudally, while the scrub nurse should stay on the back side, in front of the first surgeon (12).
Incision
In order to avoid the latissimus dorsi section the ideal monoportal incision is between fourth and sixth intercostal space, anteriorly to the muscle border and it should be at suitable distance for finger exploration. At this point is possible to coagulate and open with electrocautery the aponeurosis in order to expose the serratus muscle and open it along the direction of its fibers. Once the chest wall plane is reached, local anesthetics reinforcement is recommended. Then the superior margin of rib limiting the intercostal selected space can be cauterized progressively up to the pleural space, paying attention not to damage the underlying lung (12).
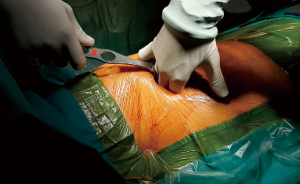
The original opening is usually enlarged by 2–3 fingers insertion (Figure 3). In order to achieve an easy rib retraction an annular wound protector and spreader (Alexis®, Applied Medical, USA) is currently used. At this point, it is possible to introduce the different surgical instruments always keeping the thoracoscope at the posterior port limit (12). Moreover, we find useful to impregnate the instruments with a fluidizing substance to facilitate multi-insertions. These last steps are generally well borne in awake patients with no need for additional local anesthesia.
Bilateral lesions can be simultaneously approached through a subxiphoid incision with the patient lying in supine position. This arciform subcostal incision allows to reach bilateral lesions located in the middle or lingular lobe or in the anterior segments of the lobes. After incising the skin, we longitudinally divide the rectum abdominis muscle along the linea alba then we open the pleural cavity immediately over the diaphragmatic anterior insertion. This operation can be facilitated through a supplemental thoracic videoport and substernal hand insertion thus allowing proper manual palpation (13).
Detection
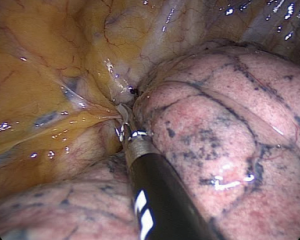
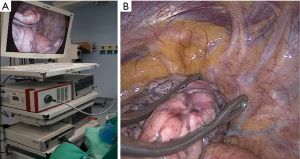
This step is the keystone of the procedure. First of all, it is necessary to explore the pleural cavity to eliminate possible pleuro-pulmonary adhesions (Figure 4). The division of these adherences allows accurate mobilization of the lung up to the lesion exteriorization. Conversely, the finding of tenacious and diffuse adhesions is a strong reason for a sudden shift to open access under general anesthesia. In case of lesions sited in the lower segments of the lung, it would be recommended to free the pulmonary ligament. This can be effortlessly dissected by energy device once infiltrated under vision the vagus nerve with 1 mL ropivacaine to prevent cough (12).
Correct patient selection should privilege lung metastases sited in submantellar position, but even apparently peripheral lesions may be difficult to detect. Therefore, several strategies have been developed, such as intraoperative echographic detection (14) and preoperatively under imaging positioned dye tracers (15) or guide-wires (16).
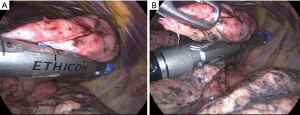
However, instrumental and digital palpations still remain the mainstay of lesion detection. Both palpations are essential and skin incision should be made considering this purpose (Figure 5). In order to better discover the lesion, we found it quite useful to measure the distance between the metastasis and the nearest fissure or other anatomical structures. Instrumental palpation is generally performed through a ring-forceps (Figure 6A,B) that allows to detect the lesion repeating action of clamping and sliding (4). We found very useful to combine the two palpations by approaching the lung to the explorative finger or, based on the lesion site, creating a counterforce of the parenchyma from the closest fissure from the basal or mediastinal faces.
If the nodule is still undetectable, after having removed Alexis retractor and increased infiltration of lidocaine you do not be uncertain to extend the incision. This widening should be preferably practiced by cutting the anterior margin of the wound and positioning a small rib retractor to allow greater breadth of the intercostal space. At this point it is possible to introduce two or more fingers (4).
Resection
This phase needs a careful monitoring by the anesthesiologist because lung traction and squeezing during stapling can induce cough reflex resulting in bleeding and parenchyma tears. Thus, at this point it is essential to reach the most profound sedation level by using propofol or remifentanil (4). The targeted metastasis can be surrounded within bended endoscopic vascular clamps (Figure 6A,B). This action facilitates the inclusion of the lesion within the stapler jaws. The stapling is performed by manual or powered linear stapler with a bite of either 45 or 60 mm (Figure 7A,B). Although a 60 mm stapler allows less firing, we prefer the 45 mm one, which results more manageable inside the pleural cavity. Once removed the specimen, it is sectioned to verify the lesion’s presence and to evaluate its macroscopic distance from resection margins and morphologic appearance (12).
As final step, a 28 Ch chest tube may be positioned at the posterior edge of the wound, but this step is not mandatory. Majority of the resections showed neither air nor fluid leak. Lung re-expansion can be accelerated by asking the subject to cough or by introducing a suction device into the pleural cavity. At this point, the patient should be awake enough to execute verbal orders (4).
Conversion to intubation under general anesthesia
Conversion to intubated general anesthesia become necessary for patient’s anesthesiologic intolerance or surgical difficulties. If possible, the operation should be interrupted and the surgical wound covered with a sterile adhesive drape. The patient should assume a supine position to allow an easier double lumen intubation but in the case of emergency intubation the anesthesiologist should be skilled at double lumen intubation even in a lateral decubitus. A compromise technique may be the intubation with the patient in semisupine decubitus (12).
Postoperative care
In the last phases of the procedure the patient is collaborative and the consciousness status is tested with the Richmond Agitation Sedation Scale, a 10-point continuous scale differentiating from combative [+4] to unarousable with no response to voice or physical stimulation [−5] (17). One hour after the surgery we use to assess patient’s recovery with a self-administered questionnaire, named “Quality of recovery (QoR-40)” that evaluates the postoperative degree of impairment (18). Patient should be timely checked from the same anesthesiologist who fulfilled the anesthesia during the surgery. Eventually, when the patient is stable the anesthesiologist allows him to return to the ward. With a post-operative chest X-ray can check the complete lung re-expansion. Eating, drinking and walking are commonly restored in the same day of the surgery as well as early physiotherapy.
Comment
Non-intubated lung metastasectomy in selected patients is as effective as traditional operations performed under general anesthesia, but compared to these presents several advantages. First, this technique results significantly reduces the global stay in operating room (4). A supplementary reduction of this parameter can be achieved by uniportal VATS under intercostal blockade and sedation. As abovementioned, this combined technique allowed to avoid the important hemodynamic consequences induced by the epidural anesthesia as hypotension and bladder block (10).
Second, the avoidance of lung collapse during the operation may decrease the risk of post-operative atelectasis and pneumonia (5), thus significantly reducing morbidity rate, recovery time, hospital stay and eventually economical costs.
Third, according to the recent Enhanced Recovery After Surgery (ERAS) guidelines the early mobilization possible after awake surgery avoids several complications including physical deconditioning, diminished muscle mass, and thromboembolism venous (19). Indeed, the non-intubated procedure allows to drinking, eating much closer to the procedure and earlier in the postoperative period than after general anesthesia. This technique significantly reduces the pre- and post-operative fast, which is currently considered an important potentially modifiable risk factor for adverse outcomes after surgery (19).
Lastly, we proved that non-intubated surgery has lesser impact on inflammatory response and lower immune-depressive effect compared to surgery under general anesthesia (20). Indeed, we have already showed a significant attenuation of interleukin 6 response in non-intubated patients (21). High level of this interleukin can lead to a transitory increment of the cortisol plasma level, which interferes with the activity of natural killer cells. Lymphocytes, and particularly natural-killer cells have a relevant cytotoxic activity against infections and tumors. Thus, the less impact on these cells could explain the reduction of postoperative infections and could suggest an improvement of the oncological outcome (21).
As limitation to the technique, we still acknowledge the difficulty of performing complete manual palpation of the lung, unless with a substernal approach. This may be a real obstacle in dealing with plurimetastatic disease with lesions sited everywhere including areas difficult to reach or resect by VATS. Once again, we recommend the proper patient’s selection for awake VATS restricted to oligometastatic, small and peripheral lesions. At this propos, we are now facilitated by the increasing definition power of the computed tomography that has significantly reduced the rate of unpredictable metastasis (3).
Conclusions
Non-intubated lung metastasectomy may achieve significant advantages in less overall operative time, lower post-operative morbidity rate and shorter hospital stay. This necessarily implies lesser economical costs. Furthermore, the milder impact on inflammatory system can significantly reduce the risk of postoperative complications. We still need a longer follow up to assess the role of non-intubated surgery on tumor progression that might be relevant in metastatic patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marcello Migliore and Michel Gonzalez) for the series “VATS in Lung Metastasectomy” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form, available at: http://dx.doi.org/10.21037/vats-2020-lm-06. The series “VATS in Lung Metastasectomy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Treasure T, Milošević M, Fiorentino F, et al. Pulmonary metastasectomy: what is the practice and where is the evidence for effectiveness? Thorax 2014;69:946-9. [Crossref] [PubMed]
- Migliore M, Gonzalez M. Looking forward lung metastasectomy-do we need a staging system for lung metastases? Ann Transl Med 2016;4:124. [Crossref] [PubMed]
- Kirienko M, Cozzi L, Rossi A, et al. Ability of FDG PET and CT radiomics features to differentiate between primary and metastatic lung lesions. Eur J Nucl Med Mol Imaging 2018;45:1649-60. [Crossref] [PubMed]
- Mineo TC, Sellitri F, Fabbi E, et al. Uniportal non-intubated lung metastasectomy. J Vis Surg 2017;3:118. [Crossref] [PubMed]
- Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761-8. [Crossref] [PubMed]
- Migliore M, Borrata F, Nardini M, et al. Systematic review on awake surgery for lung metastases. Video-assist Thorac Surg 2017;2:70. [Crossref]
- Ambrogi V, Sellitri F, Perroni G, et al. Uniportal video-assisted thoracic surgery colorectal lung metastasectomy in non-intubated anesthesia. J Thorac Dis 2017;9:254-61. [Crossref] [PubMed]
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Services, 1971.
- Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry 1983;40:812. [Crossref] [PubMed]
- Mineo TC. Epidural anesthesia in awake thoracic surgery. Eur J Cardiothorac Surg 2007;32:13-9. [Crossref] [PubMed]
- Kissin I. Depth of anesthesia and bispectral index monitoring. Anesth Analg 2000;90:1114-7. [Crossref] [PubMed]
- Ambrogi V, Perroni G, Mineo TC. Awake non intubated pulmonary metastasectomy. J Vis Surg 2019;5:38. [Crossref]
- Mineo TC, Ambrogi V, Paci M, et al. Transxiphoid bilateral palpation in video-assisted thoracoscopic lung metastasectomy Arch Surg 2001;136:783-8. [Crossref] [PubMed]
- Matsumoto S, Hirata T. Ultrasonographic evaluation of small nodules in the peripheral lung during video-assisted thoracic surgery (VATS). Eur J Cardiothorac Surg 2004;26:469-73. [Crossref] [PubMed]
- Yang SM, Ko WC. Image-guided thoracoscopic surgery with dye localization in a hybrid operating room. J Thorac Dis 2016;8:S681-9. [Crossref] [PubMed]
- Eichfeld U, Dietrich A. Video-assisted thoracoscopic surgery for pulmonary nodules after computed tomography-guided marking with a spiral wire. Ann Thorac Surg 2005;79:313-6. [Crossref] [PubMed]
- Sessler CN, Gosnell MS. The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002;166:1338-44. [Crossref] [PubMed]
- Myles PS, Weiktamp B. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth 2000;84:11-5. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold EA, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Mineo TC, Sellitri F, Vanni G, et al. Immunological and inflammatory impact of non-intubated lung metastasectomy. Int J Mol Sci 2017;18:1466. [Crossref] [PubMed]
- Vanni G, Tacconi F, Sellitri F, et al. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg 2010;90:973-8. [Crossref] [PubMed]
Cite this article as: Ambrogi V, Carlea F, La Rocca E, Mineo TC. Awake surgery for lung metastasectomy. Video-assist Thorac Surg 2020;5:35.