Is preoperative histological diagnosis in lung metastases necessary?
Introduction
Based on the widespread practice in most of the centers, preoperative histological confirmation before the lung metastasectomy is rarely performed. However, an attempt to check the appropriateness of this practice throughout the literature is not easy. In fact, no studies specifically addressed that issue, so that the above asked question can be answered only indirectly from the existing evidence or by asking this question in another way, like “what is the true value of lung metastasectomy” or “when is the preoperative histology certainly not necessary”?
There is no answer that could fit for most of the clinical scenarios. There are too many factors that influence the decision whether histological confirmation of suspected lung metastases (LM) is necessary, like the presence or absence of the known primary tumour, tumour stage and ability to control it, tumour histology, limitations of histological diagnostics, age, fitness for surgery, timing of metastases appearance, or the number and location of metastases. Furthermore, the widespread use of video-assisted thoracoscopic surgery (VATS) procedures has changed the usual delineation line between classical biopsy and therapeutic procedures.
Before discussing possible clinical situations, some common prerequisites for the lung metastasectomy with curative intent deserves to be mentioned: (I) the primary tumour must be under control or controllable; (II) no evidence about distant uncontrollable metastases; (III) the tumor must be resectable, the pulmonary reserve must be adequate; (IV) there are no available therapeutic alternatives with lower morbidity. The term “controllable” primary tumor means that the potential surgical “eradication” of the tumor may be predicted with high probability at the time of diagnosis (1).
In case of CT-PET positive nodes, routine video-mediastinoscopy before the lung metastasectomy is the usual practice in many centers (2), but is not routinely recommended in all below described clinical situations.
Solitary/multiple uni-/bilateral lung lesions in presence of known and potentially curatively treatable pulmonary or nonpulmonary primary carcinoma
Only 15% to 25% of patients with LM are appropriate candidates for surgery (3). For this reason, in this group, more important than the need to provide the preoperative histology is to perform the appropriate staging for extrapulmonary metastatic spread by means of computerized tomography (CT) of the chest and abdomen and, when necessary, by positron-emmission tomography (PET) scan and brain imaging with either magnetic resonance imaging (MRI) or CT (4).
If the appropriate staging is performed, in patients with suspected solitary LM or with a limited number of unilateral LM, the upfront metastasectomy, without previous histopathological confirmation is fully justified. This because in the reported series of metastasectomies between 1985 and 2010, 5- and 10-year survival rates were 35% to 45% and 20% to 30%, respectively. Higher survival rates for patients with single lesions have been reported as well (5).
If the suspected solitary metastasis is located in the lung contralateral to the primary tumour-bearing lung, either metastasectomy, or primary tumour resection may be done first. Beside the clear functional advantage of performing the more limited resection first, such an approach enables the detection of the synchronous primary and performing the appropriate extent of resection. If ex tempore analysis can not reliably distinguish between LM and new primary in presence of primary lung cancer with known histology, the decision about the definitive extent of resection of the LM bearing lung lobe should be postponed and performed in the second act, if necessary.
In rare patients in this group, preoperative histology of the suspected LM may be necessary in presence of more centrally located lung primary whose histology could not be established by bronchoscopy or/and mediastinoscopy and whose radiographic morphology suggests the possible small-cell type.
Lung nodules in patients with nonpulmonary cancer
In this group of patients, histological confirmation of suspected LM should be attempted whenever possible, by using all possible tools-bronchoscopy, percutaneous biopsies and VATS. This because retrospective data in populations of patients with cancer show a high frequency of benign lesions (up to 58%) and primary lung cancers (up to 50%) found after biopsies or surgery for pulmonary nodules (PN) (6). In some reports lower rates of malignant nodules were reported, like in 151 patients with extrapulmonary cancers who underwent PN biopsy, with 42% of the nodules were found to be malignant, including newly diagnosed lung cancers (7).
Concerning the prognostic factors, in a study on 228 patients with nonpulmonary malignancy who underwent a biopsy and 64% with LM, (second primary lung tumour in 26.3%, no malignancy in 9.6%), the presence of multiple PNs (>5 mm) and cavitation/necrosis were the only significant factors suggestive of metastatic disease (8). Some other factors, such as primary site, nodule size or concomitant extrapulmonary lesions have been predictive of malignancy in some studies as well (7,8).
The significance of biopsy is additionally supported by some rare reports of change in immunohistochemical profile: for example, the primary breast cancer may be estrogen receptor negative and PR negative, and the pulmonary nodule may be PR positive. This may be important in terms of the more effective treatment for breast cancer recurrence (9,10).
Concerning invasive diagnostics in this group, the specificity of LM is that some of them, despite typical ovoid-shaped radiographic aspect and intraparenchimal growth, may have a partly endobronchial growth as well. Some studies, by combining forceps biopsies with brush citology, reported the 84.2% positivity of the endobronchial biopsy with endobronchial lesions and in 60.7% of patients with normal endobronchial finding (11). Some other authors do not suggest fiber-bronchoscopy in patients with a low probability of endobronchial pathology (12). The reports of correlation between atelectasis and endobronchial metastases and a higher rate of histological diagnosis are well known since a long time ago (13,14). Metastases of colorectal carcinoma and carcinoma of the kidney were reported long ago to be associated with frequent endobronchial growth, although for the latter it was not as frequent in some series (15,16).
Transcutaneous fine needle-aspiration biopsy (FNAB) is another diagnostic tool in patients with suspected LM, in whom the diagnosis is unlikely to be obtained by bronchoscopy. The FNAB may be done either US- or CT-guided and provides samples for citology. If cutting needles are used, histological diagnosis is possible, thus improving overall diagnostic accuracy (17). By using FNAB with histological samples, owing to the accurate diagnosis of benign lesions has reduced the need for diagnostic surgery by up to 50% (18,19).
Multiple LM
The survey conducted by the European Society of Thoracic Surgeons (ESTS) showed that in the current European practice, 85% of respondents do not believe that multiple metastases represent a contraindication for metastasectomy, and none of the respondents regarded the presence of multiple metastases as an absolute contraindication (20).
The easiest scenario exists in patients with more than one suspected LM in the primary tumour-bearing lung lobe, that can be removed with the operative specimen, so that no preoperative histology of LM is necessary.
Some reports included very high numbers of metastasectomies, such as a report on laser surgery allowing minimal loss of parenchyma. In one report, in a group of 328 patients, an average of 10 lesions per patient were removed (21). In one patient with disseminated breast cancer, as many as 124 pulmonary metastases were resected.
However, the evidence suggests a correlation between the number of LM and survival. In 1995, in a study of 38 patients who had been operated on in 12 hospitals, all patients with removal of four or more pulmonary metastases had tumor progression within 16 months, whereas 50% of patients with less than four metastases were still alive without evidence of disease 5 years after metastasectomy (22).
As the number of LM reflects the degree of tumour dissemination, the histological confirmation may be appropriate if surgery for multiple suspected metastases is not an option, like in patients with numerous large metastases and high tumour grade (23). In this situation definitive nonsurgical treatment of the known primary (although itself resectable) is not indicated and some multidisciplinary teams sometimes require the histological confirmation of LM prior to start with chemotherapy protocols. VATS is usually an appropriate option in this situation.
However, the primary tumour histology and tumour grade are prognostically important. In patients with lung metastasctomies from bone and soft tissue sarcoma, around 15% of patients were reported to have more than 4 lesions, what was prognostically less favourable in comparison with solitary lesions. In addition, a worse survival was observed in a series of 214 patients when the size of LM was >2 cm (24). A disease-free interval of more than 12 months before the development of metastases was also reported a favorable prognostic factor (25).
Bilateral LM
The suspicion of bilateral LM in presence of treatable primary tumour is more challenging. The oncological benefit of bilateral metastasectomy is supported by some studies that did not confirm the worse survival after bilateral compared to unilateral metastasectomy (26,27). In this group of patients, the assessment of chances for complete resection and the functional loss prediction are priorities, rather than preoperative histological confirmation of LM.
The preoperative lung function prediction should follow the existing guidelines by using the calculation of the predicted postoperative forced expiratory volume in 1 second (ppoFEV1). Some additional informations can be obtained by using vibration response imaging (28), quantitative CT (29) and single photon emission CT (SPECT) (30). Also, when a bilateral operation is planned, a diaphragm movement assessment should not be underestimated, as recently demonstrated (31).
The operative approach may be VATS or open, either in form of bilateral thoracotomies (simultaneous/successive) or sternotomy. The suitability of subxiphoid approach for bilateral LM was also demonstrated (2). An advantage of VATS approach in these patients is the possibility to get the histological confirmation in borderline cases before the definitive decision for bilateral metastasectomy.
LM requiring extended lung resection
Nowadays a major lung resection is not considered an absolute contraindication for metastasectomy, provided that complete resection is possible (32). Extensive and combined resections can be performed with low operative mortality, morbidity and an acceptable long-term survival (33). In these patients preoperative histology is necessary whenever possible. In central tumours bronchoscopy is indicated with all available biopsy devices (biopsy forceps, brush, catheter TBNA). For peripheral tumours invading the chest wall and requiring a subsequent lobectomy with the chest wall resection, transthoracic US- or CT-guided FNAB is necessary.
Suspected metastases in presence of known, but non-resectable primary
In this group of patients, histological confirmation of suspected LM should be attempted only if clinically relevant. This because in a high proportion of these patients some forms of specific oncological treatment has already been started before the occurrence of suspected LM.
If the non-resectable primary is detected by imaging (CT, PET/CT, MRI), but for any reason without histological confirmation, a biopsy of the suspected LM is indicated by bronchoscopy, imaging-guided FNAB or by VATS, depending on the lesion(s) localization. In presence of synchronous pleural effusion, a thoracoscopic biopsy may reveal pleural carcinosis, even if not suspect on imaging, thus providing the histological confirmation of malignancy (34).
In patients who are for any reason allocated for palliative treatment for the primary tumour, histological diagnosis of LM is not necessary.
Solitary/multiple uni-/bilateral lung lesions, suspect on LM, without the known primary or after a prolonged remission
Unlike patients with known primary and multiple lung lesions, in whom biopsy is unlikely to alter the presumed diagnosis of metastases, in the absence of the known primary, the biopsy makes sense whenever possible (35). This because the radiographic aspect of slowly growing or newly developed nodules may exist in patients with benign diseases such as rheumatoid or Wegener’s or other granulomatosis, or with infections (especially in immunocompromised patients) and can be diagnosed by biopsy (36,37).
This group of patients includes those with small, sub-centimeter nodules, including ground-glass opacities (GGO) and greater nodes with diameter >5 cm or more. Despite a limited value of PET/CT in presence of nodules <8 mm, especially in pure ground-glass nodes, some recent studies have rebuilt its diagnostic value in patients with solitary pulmonary nodules. A latest meta-analysis suggested that PET/CT is still a useful method for qualitative detecting of malignant pulmonary nodules (38).
Although some cases of metastatic lung tumor showing GGOs from adenocarcinoma of the gastrointestinal tract and malignant melanoma have been reported, GGO due to a metastatic tumor is uncommon (39,40). However, this possibility should be also kept in mind. Currently, the diagnostic workup (follow up or invasive diagnostics) in presence of GGO-s is based mostly on the size of the lesion. According to Fleischner Society guidelines, the cutoff value of diameter sub-solid nodule is set as 5 mm and if solitary pure GGNs ≤5 mm, no CT follow-up is necessary, whilst multiple pure GGNs ≤5 mm should be followed-up by CT at 2 and 4 years. Initial follow-up CT at 3 months is needed and recommended to confirm persistence. The biopsy or surgical resection is recommended for persistent, dominant nodules with part-solid or solid component, especially for lesions with >5 mm solid component (41). In 2006. The NELSON trial reported about the value of volumetric measurements to identify nodules predictive of lung cancer. Nodules with a volume <100 mm3 were not found as predictive of lung cancer, whilst for nodes with a volume 100–300 mm3 the volume doubling time assessment was suggested (42). The combination of all available diagnostic tools, such as EBUS, electromagnetic navigation bronchoscopy, virtual bronchoscopic navigation, VATS and open surgical biopsies is recommended to improve the diagnostic yield for pulmonary nodules (43).
In patients with a prolonged remission after initial oncological treatment, confirmation of recurrence is frequently required in order to plan further treatment. In this situation, the diagnostic work-up is performed as previously described for clinical scenarios 1 and 3.
In the group of patients with unknown primary, VATS biopsy has some clear advantages vs. other biopsy techniques, mostly relating to some histological issues. Indeed, there are two possible scenarios in practice: (I) LM (either adenocarcinoma or other than adenocarcinoma) with microscopic features suggesting probable sites of origin; (II) LM whose morphology offers limited informations about the site of origin. For example, in a metastatic lesion, the presence of solid nests and trabeculae composed of tumor cells with limited nuclear pleomorphism, punctate areas of necrosis suggestive of comedocarcinoma, or trabeculae composed of small hyperchromatic tumor cells strongly suggests the possibility of a breast origin, particularly in a female patient (44). Or, the presence of prominent glandular formation in a metastatic tumor in bone or brain suggests the possibility of a pulmonary origin for the lesion (45). Among tumours other than adenocarcinoma listed under the scenario 1, metastatic neuroendocrine, sarcomatoid carcinomas or metastatic germ cell tumours may be encountered. The diagnosis of the letter group suggests the need to exclude primary lesions in the gonads, mediastinum, and central nervous system (46,47). The aforementioned considerations relate to surgically removed specimens and even then, it is not easy to establish the diagnosis. As the lesion removed by VATS offers a significantly greater volume of tissue vs. biopsy specimens obtained by other methods, it is clear that after VATS, the possible primary tumour is more reliably suggested, and further diagnostics more accurately directed.
Suspected LM after the initial, potentially curative resection of the known primary of any localization
In patients with suspected LM in the absence of local recurrence after the operation for extrapulmonary primary, potentially curative treatment can be planned. In this case, preoperative histology is not mandatory. Importantly, independently of presence or absence of symptoms, the local status at the site of the previously resected tumour must be checked, not only by imaging, but by endoscopy as well, whenever possible (laryngoscopy, broncoscopy, esophagogastroscopy, colonoscopy, cystoscopy etc. depending on localization).
In case of local recurrence, eventual biopsy (surgical or nonsurgical) may be required for further oncological treatment.
Some recent studies suggest the appropriatness of histological confirmation of solitary lung nodules in patients with previous malignancies in order to optimize treatment, like a study on 140 patients with prior known malignancies and lung nodules removed by VATS. In this series, only 50% of resected nodules were metastases, whilst a newly diagnosed lung primary was detected in 25% of the resected specimens. Upper lobe localization and SUVmax >2.5 were predictors of malignancy. With the exception of the smoking status, no other factors could delineate between primary lung cancer and metastases (48). These data may be of help in decision making process in patients with such a clinical scenario.
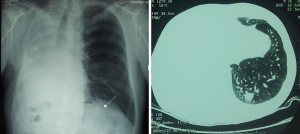
In patients with suspected ipsilateral LM after initial lobectomy or bilobectomy for primary lung cancer, histological diagnosis of the LM is not always feasible and, more importantly, not really necessary. If PET/CT indicates the presence of resectable lesion, usually located deeper in the lungs, either sublobar resection, or even completion pneumonectomy may be indicated (Figure 1).
After the initial sublobar resection, a rest-lobectomy is sometimes possible, but not infrequently with some of the broncho- or vasculoplastic procedures. In patients with a limited lung function, nonsurgical alternatives, such as stereotactic radiation therapy, are also available.
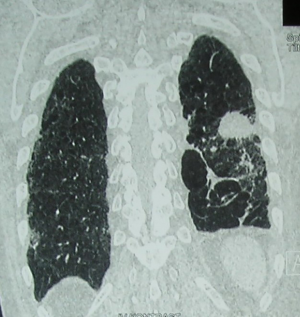
If a suspicion on LM exists after previous contralateral lobectomy, the position and motility of the both diaphragms should be checked (Figure 2).
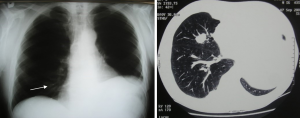
After pneumonectomy, metastasectomy is rare, but has also been reported (Figure 3). Of course, in this case functional assessment is the priority, rather than histological confirmation of suspected LM. In the four largest series ever published, only two out of a total of 65 patients underwent a lobectomy while the remaining 63 had wedges or segmentectomies (49,50).
Suspected LM after previous lung metastasectomy
In patients in this group who are candidates for re-do surgery, the histological diagnosis is usually not mandatory, because of the radiographic (CT) aspect that is usually highly suggestive of LM. The praxis of repeated metastasectomies seems to be underreported. There are no widely accepted recommendations and open surgery is performed in a majority of patients.
Rare studies on reoperations for LM reported an interesting finding: in a group of 113 patients with 2–6 lung metastasectomies, tumors initially showed a less aggressive biological pattern, followed by more malignant course, non responsive to conservative treatmen, without the increase in perioperative mortality or morbidity (51). Furthermore, tumour size, number of resections and probability of recurrence directly correlated with the number of operation, whereas an inverse correlation existed between these factors and disease free interval, that became shortened. It seems that redo metastasectomy may be appropriate for the initial procedures, whilst afterwards, because of the decrease of disease-free and overall survival, surgical therapy loses its efficacy.
In patients with a limited lung function, nonsurgical alternatives, such as stereotactic radiation therapy should be considered as well (Figure 4).
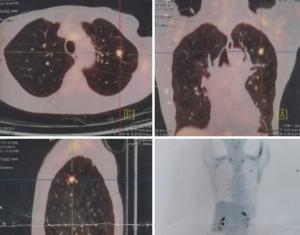
Suspected LM synchronous with solitary controllable metastases in other organs
Nowadays, the presence of simultaneous lung and liver metastases should not be considered an absolute exclusion criterion for metastasectomy. Although histological confirmation of LM in this situation is not mandatory, it can be done in suitable peripheral lesions of sufficient size. Synchronous liver metastases can be biopsied as well under US or CT guidance. In selected patients, combined one stage multi lung-liver resections demonstrated a long term survival (52). However, based on poor reported outcome, in the presence of high levels of CEA plus CA19-9 at first metastasectomy, surgery should be carefully reconsidered.
Isolated LM synchronous with solitary brain metastasis does not represent a contraindication for surgery, provided there is no other distant spread.
Conclusions
Based on the existing evidence, the histological confirmation of lung LM is not always necessary, but it may be clinically relevant in selected patients. Although there are no recommendations that could refer to most of the patients, the presented evidence could help in avoiding unnecessary biopsies, in the same time not omitting them when clinically relevant. By allocating the patients into the described clinical scenarios prior to decision about diagnostics, taking account of available technical resources and personal skills, an appropriate decision may be facilitated.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marcello Migliore and Michel Gonzalez) for the series “VATS in Lung Metastasectomy” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form, available at: http://dx.doi.org/10.21037/vats-2020-lm-01. The series “VATS in Lung Metastasectomy” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rusch VW. Pulmonary metastasectomy: a moving target. J Thorac Oncol 2010;5:S130-1. [Crossref] [PubMed]
- Mineo TC, Ambrogi V. Lung metastasectomy: an experience-based therapeutic option. Ann Transl Med 2015;3:194. [PubMed]
- Erhunmwunsee L, Tong BC. Preoperative Evaluation and Indications for Pulmonary Metastasectomy. Thorac Surg Clin 2016;26:7-12. [Crossref] [PubMed]
- Kondo H, Okumura T, Ohde Y, et al. Surgical treatment for metastatic malignancies. Pulmonary metastasis: indications and outcomes. Int J Clin Oncol 2005;10:81-5. [Crossref] [PubMed]
- Inoue M, Kotake Y, Nakgawa K, et al. Surgery for pulmonary metastases from colorectal carcinoma. Ann Thorac Surg 2000;70:380-3. [Crossref] [PubMed]
- Mery CM, Pappas AN, Bueno R, et al. Relationship between a history of antecedent cancer and the probability of malignancy for a solitary pulmonary nodule. Chest 2004;125:2175-81. [Crossref] [PubMed]
- Khokhar S, Vickers A, Moore MS, et al. Significance of non-calcified pulmonary nodules in patients with extrapulmonary cancers. Thorax 2006;61:331-6. [Crossref] [PubMed]
- Caparica R, Mak MP, Rocha CH, et al. Pulmonary nodules in patients with non-pulmonary cancer: not always metastases. J Glob Oncol 2016;2:138-44. [Crossref] [PubMed]
- Lindström LS, Karlsson E, Wilking UM, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol 2012;30:2601-8. [Crossref] [PubMed]
- Khasraw M, Brogi E, Seidman AD. the need to examine metastatic tissue at the time of progression of breast cancer: Is re-biopsy a necessity or a luxury? Curr Oncol Rep 2011;13:17-25. [Crossref] [PubMed]
- Díaz G, Jiménez D, Domínguez-Reboiras S, et al. Yield of bronchoscopy in the diagnosis of neoplasm metastatic to lung. Respir Med 2003;97:27-9. [Crossref] [PubMed]
- Argyros GJ, Torrington KG. Fiberoptic bronchoscopy in the evaluation of carcinoma metastatic to the lung. Chest 1994;105:454-7. [Crossref] [PubMed]
- Poe RH, Ortiz C, Israel RH. Sensitivity, specificity, and predictive values of bronchoscopy in neoplasm metastatic to lung. Chest 1985;88:84-8. [Crossref] [PubMed]
- Braman SS, Whitcomb ME. Endobronchial metastasis. Arch Intern Med 1975;135:543-7. [Crossref] [PubMed]
- Baumgartner WA, Mark JBD. Metastatic malignancies from distant sites to the tracheobronchial tree. J Thorac Cardiovasc Surg 1980;79:499-503. [Crossref] [PubMed]
- Noy S, Michowitz M, Lazebaik N, Baratz M. Endobronchial metastasis of renal cell carcinoma. J Surg Oncol 1986;31:268-70. [Crossref] [PubMed]
- Greif J, Marmur S, Schwarz Y, et al. Percutaneous core needle biopsy vs. fine needle aspiration in diagnosing benign lung lesions. Acta Cytol 1999;43:756-60. [Crossref] [PubMed]
- Westcott JL, Rao N, Colley DP. Transthoracic needle biopsy of small pulmonary nodules. Radiology 1997;202:97-103. [Crossref] [PubMed]
- Charig MJ, Stutley JE, Padley SPG, et al. The value of negative needle biopsy in suspected operable lung cancer. Clin Radiol 1991;44:147-9. [Crossref] [PubMed]
- Internullo E, Cassivi SD, Van Raemdonck D, et al. Pulmonary metastasectomy a survey of current practice amongst members of the European Society of Thoracic Surgeons. J Thorac Oncol 2008;3:1257-66. [Crossref] [PubMed]
- Rolle A, Pereszlenyi A, Koch R, et al. Is surgery for multiple lung metastases reasonable? A total of 328 consecutive patients with multiple-laser metastasectomies with a new 1318-nm Nd:YAG laser. J Thorac Cardiovasc Surg 2006;131:1236-42. [Crossref] [PubMed]
- van Halteren HK, van Geel AN, Hart AA, et al. Pulmonary resection for metastases of colorectal origin. Chest 1995;107:1526-31. [Crossref] [PubMed]
- Weiser MR, Downey RJ, Leung DH, et al. Repeat resection of pulmonary metastases in patients with soft-tissue sarcoma. J Am Coll Surg 2000;191:184-90. [Crossref] [PubMed]
- Choong PFM, Pritchard DJ, Rock MG, et al. Survival after pulmonary metastasectomy in soft tissue sarcomas: prognostic factors in 214 patients. Acta Orthop Scand 1995;66:561-8. [Crossref] [PubMed]
- Billingsley KG, Burt ME, Jara E, et al. Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis. Ann Surg 1999;229:602-10. [Crossref] [PubMed]
- McCormack PM, Burt ME, Bains MS, et al. Lung resection for colorectal metastases:10-year results. Arch Surg 1992;127:1403-6. [Crossref] [PubMed]
- Pfannschmidt J, Muley T, Hoffmann H, et al. Prognostic factors and survival after complete resections of pulmonary metastases from colorectal carcinoma: experiences in 167 patients. J Thorac Cardiovasc Surg 2003;126:732-9. [Crossref] [PubMed]
- Kim HK, Yoo D, Sung HK, et al. Vibration response imaging in prediction of pulmonary function after pulmonary resection. Ann Thorac Surg 2012;94:1680-6. [Crossref] [PubMed]
- Gill G, Bauer C, Beichel RR. A method for avoiding overlap of left and right lungs in shape model guided segmentation of lungs in CT volumes. Med Phys 2014;41:101908. [Crossref] [PubMed]
- Toney LK, Wanner M, Miyaoka RS, et al. Improved prediction of lobar perfusion contribution using technetium-99m-labeled macroaggregate of albumin single photon emission computed tomography/computed tomography with attenuation correction. J Thorac Cardiovasc Surg 2014;148:2345-52. [Crossref] [PubMed]
- Subotic DR, Stevic R, Gajic M, et al. Diaphragm motion and lung function prediction in patients operated for lung cancer - a pilot study on 27 patients. J Cardiothorac Surg 2013;8:213. [Crossref] [PubMed]
- Migliore M, Jakovic R, Hensens A, et al. Extending surgery for pulmonary metastasectomy: what are the limits? J Thorac Oncol 2010;5:S155-60. [Crossref] [PubMed]
- Casiraghi M, Maisonneuve P, Brambilla D, et al. The role of extended pulmonary metastasectomy. J Thorac Oncol 2015;10:924-9. [Crossref] [PubMed]
- Agarwal R, Aggarwal AN, Gupta D. Diagnostic accuracy and safety of semirigid thoracoscopy in exudative pleural effusions: a meta-analysis. Chest 2013;144:1857-67. [Crossref] [PubMed]
- Patz EF, Fidler J, Knelson M, et al. Significance of percutaneous needle biopsy in patients with multiple pulmonary nodules and a single known primary malignancy. Chest 1995;107:601-4. [Crossref] [PubMed]
- Carruthers DM, Connor S, Howie AJ, et al. Percutaneous image-guided biopsy of lung nodules in the assessment of disease activity in Wegener’s granulomatosis. Rheumatology (Oxford) 2000;39:776-82. [Crossref] [PubMed]
- Laurent F, Latrabe V, Vergier B, et al. CT-guided transthoracic needle biopsy of pulmonary nodules smaller than 20 mm:results with an automated 20-gauge coaxial cutting needle. Clin Radiol 2000;55:281-7. [Crossref] [PubMed]
- Ruilong Z, Daohai X, Li G, et al. Diagnostic value of 18F-FDG-PET/CT for the evaluation of solitary pulmonary nodules: a systematic review and meta-analysis. Nucl Med Commun 2017;38:67-75. [Crossref] [PubMed]
- Tsushima Y, Suzuki K, Watanabe S, et al. Multiple lung adenocarcinomas showing ground-glass opacities on thoracic computed tomography. Ann Thorac Surg 2006;82:1508-10. [Crossref] [PubMed]
- Yanagitani N, Kaira K, Ishizuka T, et al. Multiple lung metastases presenting as ground-glass opacities in a pulmonary adenocarcinoma: a case report. Cases J 2009;2:6910. [Crossref] [PubMed]
- Baldwin DR, Callister ME, Graham R, et al. Pulmonary nodules again? The 2015 British Thoracic Society guidelines on the investigation and management of pulmonary nodules. Clin Radiol 2016;71:18-22. [Crossref] [PubMed]
- Xu DM, Gietema H, de Koning H, et al. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer 2006;54:177-84. [Crossref] [PubMed]
- Skouras VS, Tanner NT, Silvestri GA. Diagnostic approach to the solitary pulmonary nodule. Semin Respir Crit Care Med 2013;34:762-9. [Crossref] [PubMed]
- Herbst J, Jenders R, McKenna R, et al. Evidence-based criteria to help distinguish metastatic breast cancer from primary lung adenocarcinoma on thoracic frozen section. Am J Clin Pathol 2009;131:122-8. [Crossref] [PubMed]
- Hammar SP. Metastatic adenocarcinoma of unknown primary origin. Hum Pathol 1998;29:1393-402. [Crossref] [PubMed]
- Berney DM. Staging and classification of testicular tumours: pitfalls from macroscopy to diagnosis. J Clin Pathol 2008;61:20-4. [Crossref] [PubMed]
- Berney DM, Warren AY, Verma M, et al. Malignant germ cell tumours in the elderly: a histopathological review of 50 cases in men aged 60 years or over. Mod Pathol 2008;21:54-9. [Crossref] [PubMed]
- Bellier J, Perentes JY, Abdelnour-Berchtold E, et al. A Plea for Thoracoscopic Resection of Solitary Pulmonary Nodule in Cancer Patients. Surg Endosc 2017;31:4705-10. [Crossref] [PubMed]
- Donington JS, Miller DL, Rowland CC, et al. Subsequent pulmonary resection for bronchogenic carcinoma after pneumonectomy. Ann Thorac Surg 2002;74:154-8; discussion 158-9. [Crossref] [PubMed]
- Terzi A, Lonardoni A, Scanagatta P, et al. Lung resection for bronchogenic carcinoma after pneumonectomy: a safe and worthwhile procedure. Eur J Cardiothorac Surg 2004;25:456-9. [Crossref] [PubMed]
- Mineo TC, Ambrogi V, Tacconi F, et al. Multi-reoperations for lung metastases. Future Oncol 2015;11:37-41. [Crossref] [PubMed]
- Mineo TC, Ambrogi V, Tonini G, et al. Long term results after resection of simultaneous and sequential lung and liver metastases from colorectal carcinoma. J Am Coll Surg 2003;197:386-91. [Crossref] [PubMed]
Cite this article as: Subotic D. Is preoperative histological diagnosis in lung metastases necessary? Video-assist Thorac Surg 2021;6:6.