
Computed tomography and positron emission tomography-staged cN0 non-small cell lung cancer
Introduction
Globally, thoracoscopic surgery (TS) has become increasingly accepted and widely used for the treatment of both early-stage and advanced non-small cell lung cancers (NSCLC) over the last two decades. However, skepticism persists regarding whether lymph node dissection (LND) during TS for lung cancers, especially advanced cases, is truly associated with the radical asset compared with thoracotomy. Clinically, adequate LND is crucial to preventing pathological understaging, which may lead to a worse prognosis and lack of consequent adjuvant chemotherapy. Several authors have sought a solution to this problem by comparing thoracotomy and TS with respect to pathological nodal upstaging or the number of lymph nodes after LND (1,2). However, few articles have discussed the surgical outcomes of these procedures (3).
Non-invasive methods such as chest computed tomography (CT), positron emission tomography (PET), and magnetic resonance imaging (MRI) can be used for preoperative staging evaluations. Most studies have postulated sensitivity and specificity rates exceeding 80% for a PET-CT diagnosis of suspected nodal metastasis when the two hilar and mediastinal stations were combined (4). We previously reported that the preoperative PET-CT-based prediction of metastasis to the right upper lobe lymph nodes in a case of right upper lobe NSCLC was challenging because hilar lymph nodes respond to various etiologies, such as benign pulmonary diseases (5,6).
Adequate surgical dissection of the hilar and mediastinal nodes is indispensable, and pathological nodal upstaging tends to depend on the surgical technique. Lee et al. reported that after TS lobectomy was first introduced at their institution, greater numbers of nodes and nodal stations were sampled during the later period vs. the earlier period; moreover, the authors commented on the apparent learning curve for LND during TS (3). In another report, we introduced our TS lobectomy protocol with the confronting upside-down monitor setting (7). Our procedure enables the direct manipulation of the hilar structures, and has reported lobectomy (8), LND (9) and segmentectomy with intravenous indocyanine (10).
We previously investigated the mediastinal sizes (MD) of tumors using thin-sliced CT with the mediastinal setting as a prognostic indicator, and used MD classification to determine new preoperative tumor size categories (11-13). With this study, we aimed to determine the clinical outcomes of patients with clinical stage N0 (cN0) on PET- and CT-negative, T1a–T2b and M0 disease (clinical stage IA1 to IB) NSCLC who underwent TS lobectomy with systemic or selective LND.
Methods
Patients
In this study, we retrospectively evaluated 245 patients treated at a single Japanese institution between April 2013 and December 2016. Of these patients, 2 who underwent thoracotomy conversion (0.8%) because of intraoperative pulmonary artery bleeding were excluded. The remaining 243 patients were deemed eligible, and their relevant characteristics are summarized in Table 1. The study was approved by the institutional review board of the Aichi Cancer Center, Aichi Prefecture, Japan. All patients underwent TS lobectomy with selective LND.
Full table
For all patients, potential distant metastasis was assessed preoperatively using chest CT, abdominal CT or ultrasonography, brain CT or MRI, and PET. Clinically, the mediastinal and hilar nodal status was deemed positive if the chest CT findings revealed a nodal short axis of at least 1.0 cm. When lymph node metastases were suspected in CT and PET, we performed endobronchial ultrasound-guided transbronchial needle aspiration to obtain to obtain the malignant information.
The clinical and pathological stage was assigned according to the 8th Edition of the TNM Classification of Malignant Tumors (14). Histopathological diagnoses were based on the 2004 classification of the World Health Organization classification (15).
All patients with the diagnosis as invasive cancer received CT semiannually, and patients with the diagnosis as invasive cancer added PET and brain MRI every year until the second year and in the fifth year.
Multiple-port (i.e., four-port) procedures
Our initial thoracoscopic segmentectomy and lobectomy (TSL) protocol was based on an upside-down monitor setting with four ports (7). Usually, we performed lobectomy with LND and segmentectomy, which was described previously in the context of TS with four definite ports (9). The patients were placed in the lateral recumbent position under general anesthesia. The surgeon’s left-hand 7-mm port was placed in the 3rd or 4th intercostal space (depending on the location of the upper/lower lobe) before the scapula. The right-hand 20-mm access port was placed in the 5th or 6th intercostal space at the post-axillary line. The first assistant manipulated the thoracoscope through an 11-mm port in the 3rd or 4th intercostal space above the breast. The second assistant obtained a surgical view via a 15-mm port in the 5th or 6th intercostal space at the anterior axillary line.
Statistical analyses
All computations were performed using standard SPSS v25.0 software (SPSS Inc., IBM, Chicago, IL, USA). The two groups of patients were compared using the Mann-Whitney U test. The survival rates in subgroups of patients were analyzed using the Kaplan-Meier method, and differences between groups were assessed using the log-rank test. A P value <0.05 was considered statistically significant.
Results
Patients
The characteristics of the patients are summarized in Table 1. Of the 243 patients, 118 (48.6%) were male and 125 (51.4%) were female, with a mean age of 65 years. The median follow-up duration after TS lobectomy was 42.6 months (range, 1.2–73.8 months). The NSCLCs were categorized in the following clinical stages: T1 mi, 12 cases (4.9%); cT1a, 44 cases (18.1%); cT1b, 106 cases (43.6%); cT1c, 66 cases (27.2%), and cT2a, 15 cases (6.2%). The nodes were classified by MD as 0–5 mm (n=49, 20.2%), 6–20 mm (n=153, 63.0%), and >20 mm (n=41, 16.9%), consistent with our previous reports. Of patients with node-positive (cN0pN1–2) disease, 10 (52.6%) harbored EGFR mutations, 4 (21.1%) harbored ALK mutations, and 5 (26.3%) harbored no mutations; no patient harbored a KRAS mutation.
Nodal upstaging
Overall nodal upstaging was recognized in 19 cases, including 10 (4.1%) involving mediastinal node upstaging (cN0pN2) and 9 (3.7%) involving hilar or peribronchial node upstaging (cN0pN1). In the MD subgroup, mediastinal node upstaging was observed in 0 (0%), 7 (4.6%, 7/153), and 3 cases (7.3%, 3/41) in the ≤5, 6–20, and >20 mm MD categories, respectively. However, no significant difference was observed between the 6–20 and >20 mm MD groups (P=0.48). Hilar or peribronchial upstaging was observed in 0 (0%), 7 (4.6%, 7/153), and 2 cases (4.9%, 2/41) in the ≤5, 6–20, and >20 mm MD categories, respectively. However, no significant difference was observed between the 6–20 and >20 mm MD groups (P=0.94). In the mutations, the incidence of nodal upstaging (cN0pN1–2) was higher in either EGFR or ALK [9.6% (14/146)] than in others [5.2% (5/97)], but no significant difference was found (P=0.21). However, that was significantly higher in ALK [21.1% (4/19)] than in others [4.0% (9/224)] (P<0.01).
Clinical outcomes
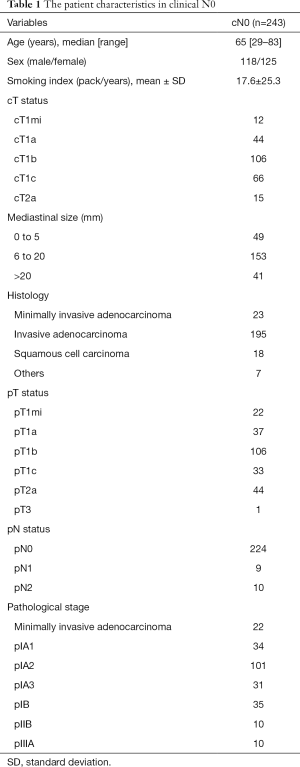
The 3- and 5-year DFS and OS rates after TS lobectomy with LND were 93.1% and 90.5%, and 97.9% and 97.9%, respectively. An analysis stratified by nodal upstaging revealed the 3- and 5-year DFS rates of 94.5% and 92.4% for cN0pN0 (n=224), 72.9% and 48.6% for cN0pN1 (n=9), and 80.0% and 80.0% for cN0pN2 cases (n=10), respectively (Figure 1A). The 3- and 5-year OS rates were 97.7% and 97.7% for cN0pN0 (n=224), 100% and 100% for cN0pN1 (n=9), and 85.7% for cN0pN2 (n=10), respectively (Figure 1B). The cN0pN0 and cN0pNpositive groups (n=19) differed significantly in terms of DFS (P<0.01) (Figure 1A), but not in OS (P=0.55) (Figure 1B).
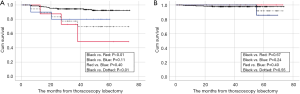
In the MD subgroup analysis, the 3- and 5-year DFS rates were 97.8% and 97.8%, 92.4% and 91.2%, 90.2% and 81.1% in the ≤5, 6–20, and >20 mm MD groups, respectively. However, no significant difference was observed between the 6–20 and >20 mm MD groups (P=0.24) (Figure 2A). A significant difference was only observed between the ≤5 and >20 mm MD groups (P=0.04) (Figure 2A).
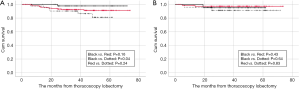
In the MD subgroup analysis, the 3- and 5-year OS rates were 95.6% and 95.6%, 98.6% and 96.2%, 97.6% and 97.6% in the ≤5, 6–20, and >20 mm MD groups, respectively (Figure 2B). However, no significant difference was observed between the subgroups (Figure 2B).
Locations of recurrent disease
Nineteen patients (7.8%) developed recurrent disease after TS lobectomy with LND. An evaluation of the locations of recurrences revealed distant, local, and both locations in 9 (47.4%), 8 (42.1%), and 2 cases (10.5%), respectively. No lymph node recurrences were identified within the resected regional nodal stations.
Discussion
This study was designed to explore the surgical outcomes of patients with cN0 NSCLC after four-port TS lobectomy. We also retrospectively investigated whether the MD affected the prognosis after thorough LND. Our findings can be summarized as follows: (I) overall mediastinal or and hilar/peribronchial upstaging was observed in 19 cases; (II) more than 70% of patients with overall nodal upstaging harbored a mutation in either EGFR or ALK; (III) multiple TS lobectomy with LND contributed to a favorable prognosis; and (IV) the MD on CT had a significant effect on DFS, but not on OS when we used this parameter to classify the surgical outcomes of cN0 patients. Despite an excess of available and competing TS approaches, no definitive strategy has been applied to determine the optimal procedure in terms of safety, expediency, reduced invasiveness and complications, and a favorable prognosis. It appears impossible to determine this optimal procedure by one expertise.
Overall nodal upstaging is a well-known surrogate marker of the effectiveness of surgical LND. In our study, overall nodal upstaging was recognized in 19 cases that were initially staged as cN0. Many authors reported nodal upstaging rates of >10% among cIA NSCLCs after lobectomy with LND, although lower rates were observed after TS than after thoracotomy (16,17). Our lower results were therefore inconsistent with those in previous reports, possibly due to the universal availability of preoperative PET for routine staging. Perigaud et al. reported that although the sensitivity for mediastinal node staging is low, the specificity is high; additionally, NSCLCs with PET-negative mediastinal nodes can be treated surgically without invasive mediastinal staging (4). Our study population comprised patients with relatively earlier-stage NSCLCs, as >80% patients had an MD of ≤20 mm. Licht et al. observed that motivation appeared to be influenced by local tradition, as well as by an individual surgeon’s skills and expertise (16). Moreover, Lee et al. reported an apparent learning curve for TS with LND when they compared the numbers of lymph nodes dissected during early- and later-period procedures (3). We expected that complete LND would at least enable the accurate clinical staging of cases with favorable outcomes.
In Japan, surgery for lung cancer accounts for >49% of all general thoracic surgeries, and TS procedures accounted for 65.6% of all lung cancer surgeries in 2012 (18). However, the definitions of TS (other than robotic surgery) vary widely. The Surgery Committee of the Cancer and Leukemia Group B reported the feasibility of a standardized and specifically defined approach to TS lobectomy (one 4- to 8-cm access and two 0.5-cm port incisions) with avoidance of rib spreading (19). In our procedure, TS lobectomy is performed using an upside-down monitor setting with four ports: one 2-cm access and three 0.5- to 1.5-cm port incisions (7). In this study, only two patients (0.8%) required conversion to thoracotomy. We also previously reported that our TS lobectomy and segmentectomy protocol is safe, with no mortality events, a reduced incidence of postoperative complications, and a reduction in hospitalization (20). We previously reported our TS with LND techniques with reference to recent studies and our experiences (9). Most significantly, MLND facilitates precise pathological staging, as demonstrated by the favorable prognosis of more than 200 patients with stage cN0 disease in this study. A large national study reported significantly higher rates of nodal upstaging when comparing TS with thoracotomy for stage I NSCLC, but did not observe a significant difference in adjusted OS (16).
The MD is used as a marker of malignant behavior and is determined by measuring the tumor size in the mediastinal window on CT. We previously reported the prognostic value of the MD (11,12). In a study of 176 small adenocarcinomas, Sakao et al. reported 5-year DFS rates of 98.1%, 71%, and 49.0% among cases with MDs of ≤5, 6-15, and >15 mm, respectively, and suggested that the MD might indicate the pleural, vessel, and pleural invasion status (11). In this study, we determined that MD was not a significant prognostic factors for DFS in patients with surgically resected cN0 LSCLC. Although Sakao et al. did not refer to OS, we considered that our results with regard to DFS were close to permittable. Sakakura et al. reported that the approximate pathological invasive nodal size was roughly the MD + 3 mm, and that MDs of 0–2 mm predicted minimally invasive adenocarcinoma (MIA) at a sensitivity of >95% (12). According to the results of our and previous studies, although MD was not an independent prognostic factor for OS, the enough patient selection as MD categorical forms could realize the acceptable patient selection and might contribute to the favorable prognosis.
This study had some limitations. First, our study featured a retrospective design and a population based on a single center. Although our study population was relatively acceptable, we could not rule out an inherent potential bias with respect to the surgical indication for TS. Therefore, we applied the MD classification to cN0 NSCLCs. We are familiar with MD evaluation and thus could avoid overestimation and measurement errors that may occur when assessing CT consolidation. Second, the surgical outcomes of the patients were unclear because of the relatively short follow-up period. However, we demonstrated equivalent OS rates at 3 and 5 years. Third, our population of patients with recurrent disease included a high proportion (73.3%) of those who harbored at least one driver mutation in ALK or EGFR. This finding was compatible with our previous report, which reported a higher incidence of detected hilar or mediastinal nodal involvement in patients with preoperative PET-negative occult lymph nodes (21). Therefore, the prognostic significance of our study of radical TS lobectomy with LND should not be evaluated solely on the basis of our results, given the potential influence of molecular targeting medications. However, it was impossible to determine this effect accurately because of the small number of recurrent cases.
In conclusion, our data demonstrate that overall nodal upstaging occurred in less than 5% of both cN0pN1 and cN0pN2 cases. The clinical outcomes after TS lobectomy with LND via a four-port upside-down monitor approach may contribute to the favorable prognosis of cN0 NSCLC staged using both CT and PET. However, we did not identify the MD as an independent prognostic factor for OS. Further investigation should be necessary.
Acknowledgments
We thank to Dr. Mingyon Mun, Cancer Institute Hospital, our TS team member in Japan, for holding this event.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Mingyon Mun) for the series “Oncological clearance of VATS lobectomy for clinical N0 non-small cell lung cancer” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2020.04.01). The series “Oncological clearance of VATS lobectomy for clinical N0 non-small cell lung cancer” was commissioned by the editorial office without any funding or sponsorship. HK serves as the unpaid editorial board member of Video-Assisted Thoracic Surgery from May 2019 to Apr 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of the Aichi Cancer Center, Aichi Prefecture, Japan (No. 2019-1-553) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Billé A, Pelosi E, Skanjeti A, et al. Preoperative intrathoracic lymph node staging in patients with non-small-cell lung cancer: accuracy of integrated positron emission tomography and computed tomography. Eur J Cardiothorac Surg 2009;36:440-5. [Crossref] [PubMed]
- Li L, Ren S, Zhang Y, et al. Risk factors for predicting the occult nodal metastasis in T1-2N0M0 NSCLC patients staged by PET/CT: potential value in the clinic. Lung Cancer 2013;81:213-7. [Crossref] [PubMed]
- Lee PC, Nasar A, Port JL, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2013;96:951-60; discussion 960-1. [Crossref] [PubMed]
- Perigaud C, Bridji B, Roussel JC, et al. Prospective preoperative mediastinal lymph node staging by integrated positron emission tomography-computerised tomography in patients with non-small-cell lung cancer. Eur J Cardiothorac Surg 2009;36:731-6. [Crossref] [PubMed]
- Al-Sarraf N, Aziz R, Gately K, et al. Pattern and predictors of occult mediastinal lymph node involvement in non-small cell lung cancer patients with negative mediastinal uptake on positron emission tomography. Eur J Cardiothorac Surg 2008;33:104-9. [Crossref] [PubMed]
- Dejima H, Kuroda H, Oya Y, et al. Evaluation of lobar lymph node metastasis in non-small cell lung carcinoma using modified total lesion glycolysis. J Thorac Dis 2018;10:6932-41. [Crossref] [PubMed]
- Mun M, Ichinose J, Matsuura Y, et al. Video-assisted thoracoscopic surgery lobectomy via confronting upside-down monitor setting. J Vis Surg 2017;3:129. [Crossref] [PubMed]
- Nakanishi K, Kuroda H, Nakada T, et al. Thoracoscopic lobectomy using indocyanine green fluorescence to detect the interlobar fissure in a patient with displaced B3 and absence of fissure: A case report. Thorac Cancer 2019;10:1654-56. [Crossref] [PubMed]
- Kuroda H, Sugita Y, Nakanishi K, et al. Lymph node dissection in the left upper lobe: clinical outcomes and surgical techniques in Japan. Mediastinum 2019;3:16. [Crossref]
- Kuroda H, Yoshida Y, Arimura T, et al. Novel development of Spectra-A using indocyanine green for segmental boundary visibility in thoracoscopic segmentectomy. J Surg Res 2018;227:228-33. [Crossref] [PubMed]
- Sakao Y, Kuroda H, Mun M, et al. Prognostic significance of tumor size of small lung adenocarcinomas evaluated with mediastinal window settings on computed tomography. PLoS One 2014;9:e110305 [Crossref] [PubMed]
- Sakakura N, Inaba Y, Yatabe Y, et al. Estimation of the pathological invasive size of pulmonary adenocarcinoma using high-resolution computed tomography of the chest: A consideration based on lung and mediastinal window settings. Lung Cancer 2016;95:51-6. [Crossref] [PubMed]
- Kuroda H, Mori S, Tanaka H, et al. Prognostic significance of combined radiologic imaging modalities for prognosis of clinical IA adenocarcinomas. Oncotarget 2017;9:10745-53. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley JInternational Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions, and International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol 2005;40:90-7. [Crossref] [PubMed]
- Licht PB, Jørgensen OD, Ladegaard L, et al. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg 2013;96:943-9; discussion 949-50. [Crossref] [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-53; discussion 353. [Crossref] [PubMed]
- Masuda M, Kuwano H, Okumura M, et al. Thoracic and cardiovascular surgery in Japan during 2012 Annual report by The Japanese Association for Thoracic Surgery Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2014;62:734-64. [Crossref] [PubMed]
- Swanson SJ, Herndon JE II, D’Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802—a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Kuroda H, Sugita Y, Watanabe K, et al. Successful postoperative recovery management after thoracoscopic lobectomy and segmentectomy using an ERAS-based protocol of immediate ice cream intake and early ambulation: a 3-year study. Cancer Manag Res 2019;11:4201-7. [Crossref] [PubMed]
- Seto K, Kuroda H, Yoshida T, et al. Higher frequency of occult lymph node metastasis in clinical N0 pulmonary adenocarcinoma with ALK rearrangement. Cancer Manag Res 2018;10:2117-24. [Crossref] [PubMed]
Cite this article as: Kuroda H, Nakada T, Oya Y, Takahashi Y, Shirai S, Matsui T, Nakanishi K, Matsusita H, Sakakura N. Computed tomography and positron emission tomography-staged cN0 non-small cell lung cancer. Video-assist Thorac Surg 2020;5:14.


