Robotic thoracic lymph node dissection for lung cancer
Introduction
Lymph node assessment is considered a critical part of oncologic staging and treatment. The number of lymph nodes removed at surgical resection, or completeness of lymph node dissection, may contribute to overall survival (OS) and recurrence free survival (RFS) (1,2). There is considerable debate on the most appropriate approach to determine which patients require a complete lymph node dissection, particularly in early-stage, clinically node-negative non-small-cell lung cancer (NSCLC) (2-4). This article will focus on the techniques for a complete lymphadenectomy during Da Vinci Xi platform (Xi) (Intuitive Surgical, Sunnyvale, CA, USA) robot-assisted lung resection.
There are slightly differing recommendations amongst societies regarding what constitutes an appropriate lymph node assessment. The National Comprehensive Cancer Network (NCCN) guidelines dictate investigation of at least three mediastinal stations (5). The International Association for the Study of Lung Cancer (IASLC) also includes ipsilateral hilar stations 10 and 11 (6). The American College of Surgeons Oncology Group (ACOSOG) and European Society of Thoracic Surgery also have featured guidelines on mediastinal lymph node assessment (7,8). The Xi provides several distinct advantages over standard VATS or open approaches for satisfying these society guidelines. These advantages are chiefly related to wristed instrument motion, use of bipolar energy, and grasping instruments in both hands to allow for safer, more efficient, cleaner, more hemostatic dissection.
Instrument selection
Instrument selection is generally surgeon-specific and is based on experience or preference, and often times is initially determined arbitrarily. We have specific preferences based on both experience and specific instrument performance metrics. Due to high case volumes, we also focus on measures that optimize efficiency. This includes minimization of instruments used, which has a halo effect on a number of processes:
- Improves sterile processing efficiency by reducing work burden.
- Improves efficiency for entire OR staff as they need to know relatively few instruments.
- Minimizes instrument exchanges which otherwise interrupt the flow of the case and contribute to instrument damage.
- Minimizes cost by all of the above.
- Instruments used:
- Zero-degree robotic thoracoscope.
- The robotic thoracoscope functions differently than traditional VATS scopes. The scope horizon and viewing angle are not independently modifiable. Because the camera and instruments all originate from the same region of the chest and converge, a zero-degree scope is generally advantageous.
- Small grasping retractor.
- This is used through the posterior port for lung retraction. Its low grip strength allows it to slide off the lung rather than easily tearing it. It is also more blunt than other long-jaw options and therefore less likely to puncture the visceral pleura.
- Cadiere forceps.
- This is used as the working instrument in the left hand, regardless of laterality. This also has low grip strength compared to similar short-jaw graspers and is similarly advantageous for that reason.
- Long bipolar forceps.
- This is used as the working instrument in the right hand, regardless of laterality. We prefer bipolar cautery because of a number of distinct advantages: (I) it allows use of a grasper in both hands; (II) allows for spreading with fine tips; and (III) energy delivery is focused and thermal spread is less.
- CO2 insufflation.
- While not classically categorized as an instrument, CO2 insufflation does provide significant advantages over its omission. (I) It provides more working room by expanding the pleural space and shifting the mediastinum. (II) The CO2 pneumodissection achieved reveals tissue planes in a superior manner. It does require awareness and experience by both the surgical and anesthesia team, however, and may result in slightly higher IV fluid administration due to the reduction in venous return. Insufflation pressure is typically set to 10 mmHg and can be adjusted lower if needed to improve hemodynamics. There is no benefit in a higher pressure, and this is poorly tolerated.
Procedure
Port placement
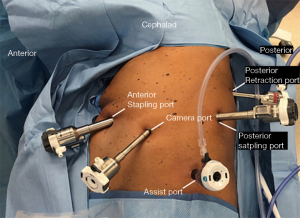
Port placement location and sequence is highly variable amongst different surgeons and institutions (9). Because of the high volume at our institution, efficiency is critical to its success and variability is undesirable where it can be eliminated. Accordingly, we have developed a port placement strategy that is consistent for all lung resections, is the same bilaterally, and is performed quickly and safely (Figure 1). The strategy, in order of placement, is as follows:
- 8 mm anterior port as anterior as possible, one intercostal space below the inframammary fold, entering the chest with a blunt introducer.
- The chest is insufflated after confirmation of intrapleural placement, and the port is exchanged for a 12 mm port.
- 8 mm posterior port placed at the edge of the innermost intercostal layer, roughly halfway between the top of the superior segment and the basilar edge of the lung.
- The remaining posterior 12 mm stapling port and anterior camera port are then placed in a manner splitting the intervening distance evenly, and generally shifted down 1–2 intercostal spaces.
- A 15 mm assistant port is placed between the camera and posterior stapling port as inferiorly as possible at the edge of the diaphragm insertion to take advantage of the wider intercostal spaces and more flexible ribs.
Station 9
The small grasping retractor is placed on the basilar surface of the lung with tips open, and the lung is retracted cephalad and laterally to expose the inferior pulmonary ligament. Alternatively, a rolled gauze can be used in the jaws. The ligament is divided up to the surface of the inferior pulmonary vein. The retracting arm is adjusted for more retraction as needed to expose the working edge of the ligament and keep it under tension. Any lymph nodes encountered within this region are removed. This dissection is essentially the same bilaterally.
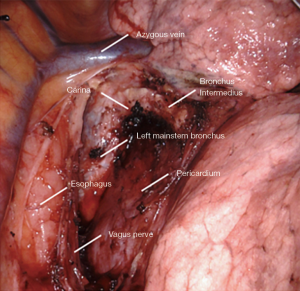
Station 7: right side
All tissue is cleared en-bloc in the subcarinal space (Figure 2). The borders are as follows:
- Carina superiorly.
- Right mainstem and bronchus intermedius on the right.
- Left mainstem on the left (deep).
- Esophagus and aorta posteriorly.
- Pericardium anteriorly.
- Superior edge of inferior pulmonary vein inferiorly.
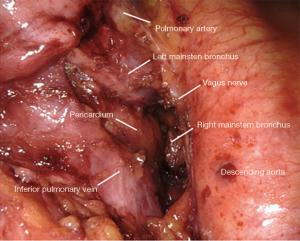
Small grasping retractor is positioned on the posterior surface of the lower lobe just cephalad to the level of the inferior pulmonary vein with the jaws open and typically facing caudally. Alternatively, a rolled gauze can be used in the jaws. The lung is retracted anteriorly and laterally, being careful not to direct too much force medially and impede venous return. The pleural reflection is opened along the posterior hilum up to the azygous vein. With gentle spreading, the vagus nerve is identified and then lifted posteriorly. It is mobilized off the underlying mediastinal structures. The station 7 lymph nodes are easily identified at this point with the vagus nerve mobilized. The surface of the station 7 lymph nodes is followed into the mediastinum, carefully cauterizing the various feeding branches and separating them from the esophagus until the left mainstem bronchus is encountered. The left mainstem is then cleared up to the carina. The inferior edge of the subcarinal tissue is then separated from the pericardium. With gentle traction on this tissue posteriorly, it can be carefully separated from the bronchus intermedius heading cephalad to the carina. This is a particular portion where bipolar energy is critically helpful. The dissection is continued cephalad toward the carina, separating the tissue from pericardium and airway until the packet is resected en-bloc.
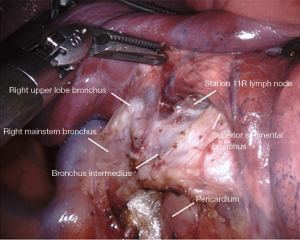
Station 11: right side
The lymph node in the bifurcation between the right upper lobe bronchus and bronchus intermedius is sometimes removed (Figure 3). Often times, it can be left in place for later easier retrieval. Dissection in this space facilitates a posterior-anterior lobectomy approach, or fissure division from an anterior-posterior approach. Boundaries include:
- Right upper lobe bronchus cephalad.
- Bronchus intermedius posteriorly.
- Superior segment bronchus caudally.
- Pulmonary artery deep.
- Lung parenchyma anteriorly.
The small grasping retractor is repositioned slightly more cephalad on the posterior surface of the lower lobe onto the superior segment, even occasionally crossing the fissure and retracting the upper lobe. Again, the jaws are open and typically facing caudally. Alternatively, a rolled gauze can be used in the jaws. The lung is retracted anteriorly and laterally, being careful not to direct too much force medially and impede ventricular filling. Lung parenchyma is carefully rolled off the posterior surface of the bronchus intermedius in an anterior direction. The membranocartilaginous junction is encountered and the cartilaginous surface of the bronchus intermedius is exposed. This is followed proximally to the origin of the RUL bronchus. There is usually a prominence of cartilage at the origin of the bifurcation at the membranocartilaginous junction. The lymph node in this space is sometimes removed, exposing the underlying pulmonary artery.
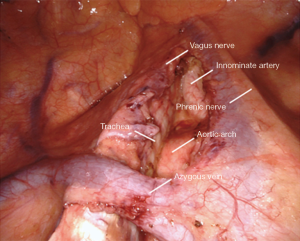
Station 2/4/10: right side
All tissue in stations 2R, 4R, and 10R is resected en-bloc (Figure 4). The boundaries include:
- Right mainstem and right pulmonary artery & truncus branch inferiorly.
- Vena cava anteriorly.
- Trachea posteriorly.
- Azygos vein superficially, left intact.
- Pericardium medially.
- Innominate artery origin superiorly.
The small grasping retractor is used to retract the lung caudally. The open jaws are placed on the mediastinal surface of the RUL and it is retracted inferiorly. Alternatively, a rolled gauze can be used in the jaws of the retractor. Any pleural attachments to the superior vena cava are carefully divided, avoiding the phrenic nerve. This may allow for further retraction and better visibility. The airway is first cleared, which facilitates identification and dissection of the pulmonary artery and truncus branch. The cava anteriorly is then carefully cleared. The azygous vein is cleared inferiorly of pleura. It is then grasped and gently elevated to facilitate clearing its deep surface along its length and then cephalad to it along the pleura. This helps when transitioning above the azygous for the paratracheal dissection. The mediastinal and lymphatic tissue is then grasped and elevated. It is carefully dissected off the airway, the pulmonary artery, and the vena cava in a cephalad direction. Once the pericardium is reached at a level just above the azygous vein, the dissection is transitioned to the paratracheal level.
The pleura is opened on the superior edge of the azygous vein and along the length of the vena cava cephalad to this, staying clear of the phrenic nerve. The resulting pleural flap is grasped and elevated. The mediastinal fat and lymphatics are cleared of this flap, facilitating identification of the vagus nerve. The vagus nerve is then carefully cleared and mobilized to avoid injury. It is critical to avoid the recurrent laryngeal nerve which originates where the vagus nerve crosses the innominate artery. The station 10 lymph nodes that were mobilized earlier are then delivered above the azygous vein and elevated. Dissection along the pericardium, airway, and vena cava is then carried cephalad along the aorta to the origin of the innominate artery, taking great care to cauterize all vessels longitudinally as this area contains dense lymphatics and is prone to lymphatic leaks. The dissection is terminated at the innominate artery origin. These stations are separated on the back table before submission for pathology.
Station 7: left side
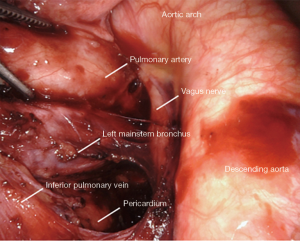
All tissue is cleared en-bloc in the subcarinal space (Figure 5). The borders are the same as from the right.
The small grasping retractor is positioned on the posterior surface of the lower lobe just cephalad to the level of the inferior pulmonary vein with the jaws open and typically facing caudally. Alternatively, a rolled gauze can be used in the jaws. The lung is retracted anteriorly and laterally, being careful not to direct too much force medially and impede ventricular filling. The pleural reflection is opened along the posterior hilum as high as possible around the aortic arch. The station 7 lymph nodes are not as easily identified on the left side. Dissection is carried along the superior edge of the inferior pulmonary vein down to the pericardium in the space between the inferior pulmonary vein and the left mainstem bronchus. The superior edge of the inferior pulmonary vein is cleared, then the surface of the pericardium and the bronchus are cleared simultaneously. Posteriorly, the vagus nerve is lifted and gentle spreading helps identify and separate the esophagus from the mediastinal lymphatic tissue. The left mainstem is then cleared up to the carina. The packet is then dissected from pericardium and airway until the packet is separated en-bloc.
Station 10: left side
All tissue on the posterior surface of the pulmonary artery cephalad to the bronchus is cleared (Figure 6). Often, station 10 lymph nodes can be identified under the pleura, but sometimes careful dissection is required. Tissue on the superior edge of the bronchus is opened and dissected until the surface of the pulmonary artery is encountered. The PA is then dissected along its exposed length. The vagus nerve is again mobilized away from the pulmonary artery. The lymph nodes encountered here are lifted off the PA and separated. This also allows dissection along the pulmonary artery in the posterior aspect of the fissure, where the last posterior segmental arterial branch to the upper lobe can be identified. This facilitates later fissure division.
Station 5: left side
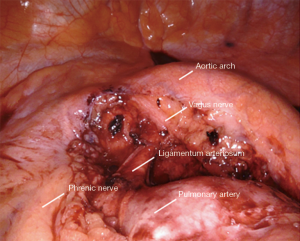
All tissue in the aortopulmonary window is removed en bloc (Figure 7). The boundaries include:
- Aortic arch lesser curve superiorly/posteriorly.
- Pulmonary artery inferiorly.
- Phrenic nerve anteriorly.
- Ligamentum arteriosum and pericardium medially.
The small grasping retractor is placed on the mediastinal surface of the left upper lobe with jaws open and the lung retracted inferiorly, taking care to avoid excessive medial force and impeding ventricular filling. Alternatively, a rolled gauze can be grasped within the jaws. Dissection from the prior station 10 dissection is continued along the pulmonary artery on its surface. The pleura is opened along the pleural reflection anteriorly to the phrenic nerve. The pleura is then elevated and the mediastinal fat and lymphatic tissue is cleared off the deep surface of the pleura. The phrenic nerve is identified, carefully separated and preserved. The lesser curve of the aortic arch is then carefully cleared, where the vagus nerve is identified and left in place. This nerve is carefully avoided to prevent injury to the recurrent laryngeal nerve. The lymphatic tissue and fat are then elevated off the pulmonary artery and the ligamentum, carrying the dissection anteriorly to the phrenic nerve where the dissection is terminated.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Natalie S. Lui and Sean C. Wightman) for the series “Robotic Surgery for Lung Cancer” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2020.01.03). The series “Robotic Surgery for Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. DSD is a proctor for Intuitive Surgical, Inc. and he reports personal fees from Intuitive Surgical, Inc., from null, outside the submitted work; WBT is an instructor and observation site administrator for Intuitive Surgical, Inc. and he reports personal fees from Intuitive Surgical, Inc., outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang W, Chen D, Xi K, et al. Impact of Different Types of Lymphadenectomy Combined With Different Extents of Tumor Resection on Survival Outcomes of Stage I Non-small-cell Lung Cancer: A Large-Cohort Real-World Study. Front Oncol 2019;9:642. [Crossref] [PubMed]
- Hughes MJ, Chowdhry MF, Woolley SM, et al. In patients undergoing lung resection for non-small cell lung cancer, is lymph node dissection or sampling superior? Interact Cardiovasc Thorac Surg 2011;13:311-5. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Mitsos S, Panagiotopoulos N, Patrini D, et al. Is systematic lymph node dissection mandatory or is sampling adequate in patients with stage I non-small-cell lung cancer? Interact Cardiovasc Thorac Surg 2019;28:550-4. [Crossref] [PubMed]
- Ettinger DS, Kris MG. Update: NCCN non-small cell lung cancer clinical practice guidelines. J Natl Compr Canc Netw 2004;2:S9-13. [PubMed]
- Vallières E, Shepherd FA, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:1049-59. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Number of lymph nodes harvested from a mediastinal lymphadenectomy: results of the randomized, prospective American College of Surgeons Oncology Group Z0030 trial. Chest 2011;139:1124-9. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Oh DS, Tisol WB, Cesnik L, et al. Port Strategies for Robot-Assisted Lobectomy by High-Volume Thoracic Surgeons: A Nationwide Survey. Innovations (Phila) 2019;14:545-52. [Crossref] [PubMed]
Cite this article as: Demos DS, Tisol WB. Robotic thoracic lymph node dissection for lung cancer. Video-assist Thorac Surg 2020;5:17.