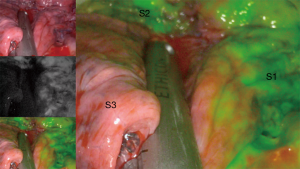
Tips and tricks in uniportal anterior segmentectomy (S3) of the right upper lobe
Introduction
With minimal-invasive pulmonary segmentectomies on the rise, more challenging and technically demanding segmentectomies are being performed (1). In the following article we describe the key steps of uniportal VATS segmentectomy of the right upper lobe anterior segment (S3). The main difficulties are the access to the bronchovascular structures of the segmental hilum, which needs most of the times a separation of the S3 from the middle lobe (2-4) and the accurate identification of the intersegmental plane between S3 and the remaining upper lobe.
The single-port technique for thoracoscopic surgery was first described for diagnostic purposes (5) and a few years later the first case of a single port lobectomy was published (6). Through its minimal invasiveness and certain advantages in geometry and exposition, the method has been gaining ground ever since. At the end of 2014, we began performing major lung resections by means of uniportal VATS at our institution. Since the beginning of performing uniportal VATS segmentectomies in April 2015 and until May 2019 we have performed more than 260 segmentectomies. We started using near-infrared fluorescence with indocyanine green as an adjunct to identify the intersegmental plane in mid-2018 (7).
Among those procedures were 15 resections of the anterior segment of the right upper lobe. In 12 cases, the patients underwent surgery due to suspicion or diagnosis of primary lung cancer, i.e., non-small cell lung cancer (NSCLC), and the remaining three were operated on due to centrally located metastasis of extrathoracic malignancies. Median operation time was 150 minutes (range, 100–252 minutes) and the perioperative outcome was similar to other segmentectomies. In this paper we describe our technique and some tips and tricks on how to perform a uniportal anatomical resection of right segment 3.
Patient selection and workup
During the past decade, there has been a rising proportion of small pulmonary lesions being radiologically detected. A great fraction of these pulmonary lesions cannot be further assessed through bronchoscopy or transthoracic puncture because of their size, localization or accessibility. For such cases, a minimally invasive segmentectomy can serve diagnostic and therapeutic purposes simultaneously.
Regarding suspected or confirmed NSCLC especially, segmentectomy needs further planning and evaluation for feasibility (8). “Intended” segmentectomy can be offered to patients with small tumors (<2 cm) and peripheral localization without compromising long term survival in comparison to lobectomy with the advantage of a better quality of life (9). For patients with poor lung functions, synchronous or metachronous lesions or concomitant malignancies, “compromised” segmentectomy can be an option even for larger tumors.
It cannot be repeated enough that minimally invasive lung segmentectomy requires perfect knowledge of the anatomy (10). In addition to usual preoperative staging investigations for NSCLC, a thin layer contrast-enhanced computed tomography of the chest is mandatory to plan the segmentectomy in terms of vascular variations and intersegmental plane. 3D modeling may be helpful if not familiar with the lecture of axial, coronal and sagittal reconstructions (11).
Perioperative setting
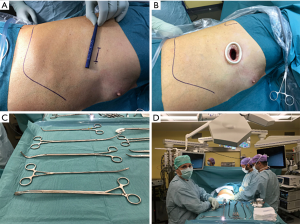
The operation is performed under general anesthesia and a double-lumen endotracheal tube is used to allow single-lung ventilation. The patient is done in the left lateral decubitus and a 3–4 cm incision is planned in the 4th intercostal space (Figure 1). The surgeon and the assistant are standing on the front of the patient with the scrub nurse being on the opposite side (Figure 1). A wound retractor is used to avoid smearing of the scope (Figure 1). Use of a high definition camera with near-infrared fluorescence mode is very helpful. Beside dedicated instruments (Figure 1) we use the electrocautery hook for most of the dissection. We use motorised staplers for bronchovascular structures and parenchyma. Energy devices coupled to hemostatic clips are an alternative for small vessels and allow a lymphadenectomy with less oozing.
Procedure
First step after entering the thoracic cavity is to check for adhesions and unexpected findings. An intercostal block with 20 mL of ropivacaine 0.75% is then performed.
Opening of the horizontal fissure
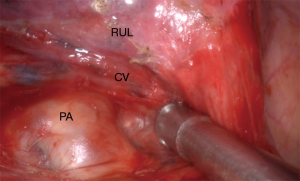
The bronchovascular structures of the pedicle of S3 are most often difficult to access. The horizontal fissure is usually fused. The first step will hence be to open the horizontal fissure in a tunnel technique. We perform this in five steps (Video 1). The dissection of the oblique fissure is first and allows revealing the pulmonary artery, which is prepared centrally until identifying the crossing central vein (Figure 2). This move prepares the exit point for the stapling device, facilitating the separation of the horizontal fissure later and is different to techniques already described (4). As a second step the mediastinal pleura is opened to reveal the middle and upper lobe veins. The first branch of the central vein is V3b, which can have a common rise with V3a like in this video. V3b can be separated at this stage if visibility is not optimal and the main pulmonary artery cannot be identified between middle and upper lobe veins. After identifying the pulmonary artery and its branch to the middle lobe (A5), the fourth step would be to use a dissector and follow the central vein in the already opened oblique fissure. Be careful not to harm A4 (if no common origin with A5) and A2 during this step. An ascending A3 is rather seldom. Finally, with or without the help of a silastic sling, you can separate the horizontal fissure with a stapling device.
Identification and section of the bronchovascular pedicle of the segment
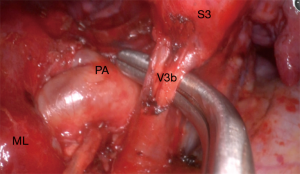
Once the horizontal fissure has been opened, the central vein lies free on its whole course, revealing its tributaries to segment 3, V3b medially and V3a more laterally (Figure 3). The veins can now be separated and the A3 artery shows up as a branch of the truncus anterior, crossing behind the preserved V1b (Video 2). The subdivision of A3 in a medial A3b and lateral A3a is variable. At this stage, a thorough lymphadenectomy (level 12 and 13) is usually necessary to reveal the borders of the A3 and the B3 bronchus. These structures need some dissection before the stapler can be slipped around and a silastic sling would be helpful. Bronchoscopy or lung inflation is advised for control of correct positioning on B3 before firing the stapler.
Parenchymal division along the intersegmental plane
The division of the parenchyma along the intersegmental plane is not trivial. We advise to lift the bronchial stump with a grasper and free it from the surrounding tissues through blunt dissection in order to gain some space and to make sure that all bronchovascular stumps are within the resected segment. Since the V1b and V2c veins determine the boarders to S1 and S2 respectively, we slip the stapler along them (Video 3). The intersegmental plane can now be precisely determined using the classic inflation/deflation line or by near-infrared fluorescence with ICG (Figure 4). The advantages of the latter method is avoiding inflating the lung, which can take some time to deflate again, and permit a more accurate delineation of the intersegmental margin. Finally, we transect the parenchyma in different directions, forming the “Mercedes Star” sign at the end. The advantages of this way of stapling are: less tension on the stapler line and preserving of the anatomy of the remaining upper lobe (2-4).
The specimen is then retrieved in a bag. Systematic mediastinal lymph node dissection (stations 2, 4, 7, 8 and 9) is completed at this point. We recommend performing an underwater test to check for air leakage. Finally, a 24 Charriere chest tube is placed through the dorsal corner of the uniportal access which is closed in a muscular and skin layer by a continuous absorbable sutures (Figure 5).
Post-operative management
After the operation, the patient is transferred to the recovery room for a couple of hours (2–4 hours) until he is fully awake. By uneventful monitoring and after mobilization, the patient is taken back to the ward. The chest tube can be removed in the absence of air leak and a 24-h serous output of less than 200 mL. In this case, the chest tube was removed at postoperative day 1 and the patient was discharged on day 4. The recovery was uneventful.
Tips, tricks, and pitfalls
- Five steps to open the horizontal fissure (
Video 1): - Dissection of the pulmonary artery in the oblique fissure and identification of the crossing central vein (exit point);
- Hilar preparation with identification of the middle and upper lobe vein and its central branch;
- Identification of the main pulmonary artery and the middle lobe artery (A5) between the middle and upper lobe veins;
- Tunneling along the central vein not harming middle lobe arteries and ascending A2;
- Division of the horizontal fissure with a stapling device.
- Dissection and preparation of segment hilum (vein, artery, and bronchus,
Video 2). - Thorough lymph node dissection facilitates the exposure of A3 and B3.
- Preservation of the V1b as the intersegmental border between S1 and S3.
- Division of the parenchyma (intersegmental plane) through stapling in different directions (Mercedes Star) to reduce the tension on the staple line and preserve the anatomy of the remaining right upper lobe (
Video 3).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Hitoshi Igai) for the series “Uniportal VATS Segmentectomy” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2020.01.05). The series “Uniportal VATS Segmentectomy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Igai H, Kamiyoshihara M, Yoshikawa R, et al. The safety and feasibility of thoracoscopic uncommon pulmonary segmentectomy. J Thorac Dis 2019;11:2788-2794. [Crossref] [PubMed]
- Lutz JA, Dorn P, Schmid RA, et al. Upper lobe anterior segment (S3): technique of fissureless uniportal VATS segmentectomy. J Thorac Dis 2018;10:3797-9. [Crossref] [PubMed]
- Lutz J, Seguin-Givelet A, Gossot D. Thoracoscopic anterior segmentectomy of the right upper lobe (S3). J Vis Surg 2018;4:183. [Crossref]
- Soultanis KM, Gonzalez-Rivas D. Uniportal video-assisted anterior upper lobe anatomic segmentectomy S3: a technical description. J Vis Surg 2018;4:140. [Crossref]
- Rocco G1. Khalil M, Jutley R. Uniportal video-assisted thoracoscopic surgery wedge lung biopsy in the diagnosis of interstitial lung diseases. J Thorac Cardiovasc Surg 2005;129:947-8. [Crossref] [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Guigard S, Triponez F, Bédat B, et al. Usefulness of near-infrared angiography for identifying the intersegmental plane and vascular supply during video-assisted thoracoscopic segmentectomy. Interact Cardiovasc Thorac Surg 2017;25:703-9. [Crossref] [PubMed]
- Ueda K, Tanaka T, Hayashi M, et al. What proportion of lung cancers can be operated by segmentectomy? A computed-tomography-based simulation. Eur J Cardiothorac Surg 2012;41:341-5. [Crossref] [PubMed]
- Bilgi Z, Swanson SJ. Current indications and outcomes for thoracoscopic segmentectomy for early stage lung cancer. J Thorac Dis 2019;11:S1662-S1669. [Crossref] [PubMed]
- Nomori H, Okada M. Illustrated Anatomical Segmentectomy for Lung Cancer. Tokyo: Springer, 2011.
- Seguin-Givelet A, Grigoroiu M, Brian E, et al. Planning and marking for thoracoscopic anatomical segmentectomies. J Thorac Dis 2018;10:S1187-S1194. [Crossref] [PubMed]
Cite this article as: Gioutsos K, Lutz JA. Tips and tricks in uniportal anterior segmentectomy (S3) of the right upper lobe. Video-assist Thorac Surg 2020;5:15.