Non-intubated VATS for the management in primary spontaneous pneumothorax
Introduction: historical perspective and present
Primary spontaneous pneumothorax (PSP) usually appears in young patients without comorbidity. Its recurrence is from 20% to 60%. Its reported incidence is 7 to 28/100,000 per year in men and 1.2 to 6/100,000 per year in women (1).
Thoracoscopic bullectomy under intubation [double-lumen tube (DLT) or simple tube (ST) with bronchial blocker] and general anesthesia (GA-VATS) has traditionally been the standard treatment for persistent or recurrent primary pneumothorax (PSP) (2-6). Ideally, bullectomy should be follow by some pleurodesis technique (3-5). In addition, it is well known that traditional thoracotomy approach can lead to higher morbidity than VATS (6,7), and has no impact in the follow-up results.
Although the evident benefits of GA-VATS comparing to thoracotomy in terms of morbidity, this is not uneventful approach. Intubation (DLT or ST with bronchial blocker) during GA-VATS has been related with sore throat, hoarseness, and even tracheal laceration has also been reported after insertion of a DLT (7,8). Diaphragm relaxation provokes alteration in the ventilation/perfusion (V/Q) matching (9). On the other hand, keeping diaphragm motion during awake procedures avoiding muscle relaxation (Figure 1), preserves the compliance in the dependent non-operative lung, which in addition gravity minimizes the disruption in the match of V/Q, compared to GA (11).

In addition, it is well known that mechanical ventilation can produce barotrauma, volutrauma, atelectrauma and proinflammatory mediators release, increasing morbidity and mortality (9). Also, volatile anaesthetics used in GA have been reported to inhibit hypoxic pulmonary vasoconstriction (HPV), leading to low compensation shunt effect (12,13).
These facts made the surgical and anaesthetic community pay attention to develop non-intubated VATS (NI-VATS) treatments for PSP treatment, with the aim of avoid the deleterious effects associated with standard GA-VATS PSP treatment. Nevertheless, in the last years, NI-VATS procedures have shown encouraging results in the treatment of PSP and other pulmonary procedures (wedge resections, lobar or sublobar anatomical resections, thymectomies…) (9).
The aim of this paper is to collect the evidence about NI-VATS PSP treatment and summarized our experience developing a NI-VATS program for treatment of PSP since 2013. A PubMed bibliographic search was made using these terms: “non intubated”, “awake”, “VATS”, “primary spontaneous pneumothorax”, “bullectomy”, “pleurodesis”.
NI-VATS strategies in PSP treatment: locoregional anesthesia strategies in PSP treatment
Locoregional strategies as thoracic epidural anaesthesia (TEA) in NI-VATS protocols are well described since 2011 by Chen and colleagues (14). In addition, TEA NI-VATS protocols have been generally stablished for non-small cell lung cancer treatment (NSCLC) (14-17). Note there is a lack of prospective randomized studies comparing exclusively surgical treatment for primary spontaneous pneumothorax between NI-VATS and GA-VATS patients, so solid evident is limited and prospective randomized studies are needed. The evidence level reaches at most level 2 (cohort or case/control studies with bias risk). This poorness of trials impedes to settle strong recommendations, which are limited to a D degree of the Scottish Intercollegiate Guidelines Network (SIGN) (18).
In 2015 Li et al. (19) presented the first descriptive study including 32 patients of PSP treated by NI-VATS bullectomy by using epidural catheter and sedation (without intubation). With the limitations of a descriptive study, the results were similar to standard GA-VATS approach for PSP treatment (3,19). The average time of surgery was 49 min with postoperative feeding time of 6 h, mean postoperative chest tube drainage was 19,3 hours and hospital stay was hours 41,6 hours respectively. Two patients described pain was moderate, while 30 patients describe pain as mild. In 14.5 months follow up no recurrences of pneumothorax were found.
Guo et al. 2016 (20) presented a cohort of 37 patients undergoing bilateral bullectomy by using TEA NI-VATS approach (n=15) or GA-VATS approach (n=22). Time of surgery, blood loss and intraoperative lowest oxigen saturation level were similar between groups. Perioperative results as postoperative chest tube time, hospital stay and surgical complications were also comparable in both groups. In addition, no recurrence differences were found. However, anaesthesia cost in NI-VATS group was significantly lower (P=0.016).
Also the group of Guo et al. 2016 (21) described a single-institution retrospective analysis comparing the results of 240 patients that received TEA-NI-VATS bullectomy (n=140) and local anesthesia (LA) NIVATS bullectomy (n=100). In the TEA-NI-VATS group epidural catheter was placed into T7-8 or T8-9 with fractioned injection of 10–15 mL 0.375% ropivacaine with the aim to reach anaesthesia level between T2 and T10 before surgery. No differences in postoperative complications, surgical duration, estimated blood loss, peak EtCO2 and lowest intraoperative SpO2 level were found between both groups, so authors conclude NI-VATS bullectomy by using TEA or LA is feasible and safe.
Hwang et al. 2018 (8) published the only one prospective, randomized, double-blinded, parallel trial comparing LA-NI-VATS vs. GA-VATS for PSP treatment in two groups of 21 and 20 patients respectively (8). The results showed that the times for anesthesia, operation and emergence were significantly shorter in LA-NI-VATS than GA-VATS treatment for SPS and that the incidence of sore throat were significantly lower in NI-VATS group with no other significant difference in the adverse events of the two groups.
In our experience the most commonly locoregional anesthesia techniques used while NI-VATS surgery for PSP are epidural anesthesia, intercostal blocks (IB) and local anesthesia (8,22,23). At the beginning of our NI-VATS program in 2013 we started by using thoracic epidural catheter (Figure 2). Later, we began to keep experience and confidence with intercostal block, and nowadays we routinary insert 1 to 1.5 cc of bupivacaine 0.5% in each intercostal space (from 2nd to 7th), at the beginning and at the end of the surgery.
However, due to well known possible side effects of epidural catheter (dural puncture headache, epidural bleeding, hypotension, infection or spinal cord injury) we switch our initial NI-VATS protocol and from 2015 for either NI-VATS or GA-VATS procedures we routinary perform LA-NI-VATS and IB under direct thoracoscopic vision (Figure 3) avoiding TEA. Nowadays we are analysing our data of NI-VATS programme from 2013 to 2019 but preliminary results showed no significant postoperative differences between TEA and IB group, having shorter operative room time in IB group and similar pain control.

NI-VATS strategies in PSP treatment: cough reflex and vagal block
Cough reflex is probably the main concern when starting to perform NI-VATS procedures so mediastinal movement can increase de risk of major bleeding while dissecting vascular structures and increase intraoperative time (23). In young patients as usually in PSP cough reflex can be increased, so an adequate inhibition of the reflex is needed to perform de surgical technique in a safe way.
To avoid cough reflex, we begin the surgery by doing vagal blockage. To achieve good vagal blockage, we insert 2–3 cc of 0.5% bupivacaine in the right paratracheal area or in the left aortopulmonary window (Figure 3). Vagal block ensures cough abolition during 12 h so surgical treatment of PSP can be performed safely. In our experience, vagal block should be completed before initiating pulling manoeuvres in order to decrease cough reflex triggering (Figure 4) (23).

Oxygenation
Due to patient’s condition (young, BMI <25, no comorbidity…) and short operative time no support for oxygenation is usually needed in a NI-VATS bullectomy for PSP treatment. However, when needed, other oxygenation dispositives can be used (facial mask, oropharyngeal cannula, or high-flow oxygen nasal prongs can be used) (Figure 5) (23).
A well-known respiratory problem in NI-VATS procedures is hypercapnia when operative time is prolonged. As bullectomy is a quickly procedure we didn’t face with hypercapnia in NI-VATS PSP treatment. If severe hypercapnia, we recommend to decrease propofol infusion and use oxygenation dispositive as facial mask until patient recovers standard parameters. If hypercapnia is not controled, conversion to tracheal intubation and mechanical ventilation should be considered in order to preserve patient safety (12,14,22,23).
Selection criteria
In 2013 during development of our NI-VATS research program we set the inclusion and exclusion criteria described in Tables 1,2 (14,26). Criteria are common for all NI-VATS procedures (bullectomy, wedge, anatomical resections…). Most common contraindications in our environment are obesity, anatomic difficulties (uncomplete fissures, bronchovascular invasion…), previous thoracic surgery, coagulation disorders, extensive pleural adhesions that enlarge surgical time, and T4 lung cancer patients.
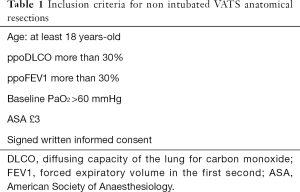
Full table

Full table
One of the best problems when starting NI-VATS programme is to find and adequate cohort of patients that fulfil the selection criteria. This aim, is easier in PSP patients, so they are young, with lower BMI and usually less comorbidities or unfavourable anatomy, that also are most common contraindications to perform NI-VATS procedures We suggest, PSP patients, as a very good cohort for start performing NI-VATS procedures, firstly, because we can easily find patients that fulfil de selection criteria. Secondly PSP NI-VATS surgical treatment is technically easily reproducible for a previously VATS experienced surgeon, and may help to acquire confidence and skills (vagal block, lung parenchyma slow movement avoiding cough stimulation, deal with diaphragmatic motion) progressively through NI-VATS approach.
Standardized NI-VATS flowchart protocol for PSP treatment
Since 2013 we have developed the following NI-VATS protocol) for PSP treatment (Figure 6).
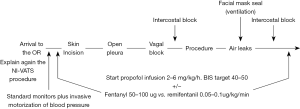
- Previous patient instructions: surgeon and anaesthesiologist should explain again to the patients the particular characteristics of NI-VATS procedure, specially about the possibility of feeling some degree of dyspnoea in case he/she is not deeply sedated in some part of the procedure.
- Standard monitoring including: electrocardiogram, oxygen saturation measured by pulse-oximetry, peripheral venous access, respiratory rate plus invasive blood pressure monitoring using the radial artery.). The anaesthetic depth is monitored through bispectral index (BIS) value, and usually kept between 40 and 60 (15). For PSP treatment, usually urinary catheter is not needed.
- Premedication: usually with 1 to 3 mg of midazolam and 50 to 100 µg of fentanyl.
- Locoregional anaesthesia: local 2% lidocaine around the surgical wound area and routinary 2nd to 7th intercostal nerves block under thoracoscopic vision with 1–1.5 mL bupivacaine 0.5% per intercostal space at the beginning and the end of the surgery.
- Sedation: In our experience, the use of a propofol infusion (2–4 mg/kg/h) plus the regional block is a good combination. Adding opioids as fentanyl (50–100 µg) is reserved to control the respiratory rate. Remifentanil can also be used, but the infusion is associated with severe cases of hypercapnia (PaCO2 >100 mmHg) in longer procedures, nevertheless, that is a very uncommon scenario while PSP treatment.
- Respiratory rata monitoring: it is important to balance respiratory rate, so in our experience a low respiratory rate, it is associated with uncontrolled mediastinal movement, that can lead to technical difficulties during procedure. Nevertheless, respiratory rate should be balance, in order to avoid excessive respiratory depression but trying to get a “static” mediastinum if possible.
- Air leaks: we complete air leak test by using facial mask in order to facilitate reexpansion and find air leak if present.
- Awakening: after chest tube is placed in the cavity wound is closed and the propofol infusion is stopped. In routinary conditions, oral intake and walking begin within the next 6 h.
Surgical technique
NI-VATS surgical technique is very similar to the conventional VATS with some specific details. Since our team routinely performs larger uniportal VATS surgery since 2011 we also perform NI-VATS procedures through this approach. In any case, the protocol is similar for those teams that prefer biportal or multiportal approach.
As a first step, we infiltrate local anaesthetic in the wound. Then we perform a 3–4 cm incision in the 5th intercostal space. If the lung is free of adhesions, we routinely perform the vagal block by infiltrating approximately 2–3 cc of bupivacaine in the areas previously described. If technically possible, we always recommend performing vagal block, however sometimes and depending on the experience of the team, in patients with very localized lesions and very short-time surgeries it is possible to perform the procedure quickly and safely without vagal block. To complete intercostal blockage we insert 1–1.5 cc of 0.5% bupivacaine in each intercostal space (usually from 2nd to 7th) (Figure 7). After that, bullectomy is achieved by using endostaplers (Figures 8,9), and then talc pleurodesis of the entire thoracic cavity is performed (Figure 10).




Ethical aspects: implementation of NI-VATS programme
As the traditional VATS treatment for PSP is well stablish and the evidence about NI-VATS PSP treatment is still very limited we recommend to start NI-VATS procedures for PSP treatment under the implementation of NI-VATS Research Program approved by the Hospital Ethics Committee. Ideally the NI-VATS surgical schedule must be carried on strictly following the inclusion and exclusion criteria stablished in the literature (12,14-17). We also recommend that patients should read and sign a specific informed consent for NI-VATS procedures and a patient information document with the anaesthetic and surgical model at least 24 hours before the procedure. In our experience the programme will be easier and safer if it is developed by professionals that have already completed the learning curve for VATS major procedures.
Discussion
NI-VATS treatment of PSP seems to be a safe and reproducible procedure in order to search for the less invasive surgical and anaesthetic approach. Despite the encouraging results of this novel technique, there is a lack of prospective randomized studies, so the evidence is limited and this fact should make us reflect (8,19,22).
In addition, the strongest evidence on the NI-VATS approach compared to the conventional VATS approach has been generated for the treatment NSCLC (14-17,23), with only one prospective randomized study comparing specific PSP treatment outcomes of NI-VATS vs. GA-VATS (8).
Technically, the surgical treatment of PSP is quite similar comparing to wedge resections for treatment of other pathologies (NSLC, metastasis, undiagnosed pulmonary nodules). Although it is true that the role of wedge-type procedures has been tested using the NI-VATS approach, proving to be safe and reproducible (14-17), one of the main problems of the groups that initiate NI-VATS programs is to obtain an acceptable amount of patients that meet the selection criteria, which in many cases, given the age and comorbidity of the patients is highly difficult.
Being technically similar to a wedge procedure for pulmonary nodule resection, the PSP surgical treatment by using NI-VATS approach is quite convenient as model to start an NI-VATS program. It has different advantages, among which stand out the middle difficulty of the procedure (preferably we recommend to start NI-VATS program selecting procedures with increasing progressive difficulty) as well as the ease to obtain patients that meet the selection criteria. In this case, patients with PSP have very favourable phenotype to be NI-VATS candidates, given that they are young, thin, and generally without other comorbidity (4).
In addition, is remarkable that in NI-VATS PSP treatment it is exceptional face with an emergent situation. NI-VATS procedures can find two big types of emergent situations, major bleeding and the need to reconvert to intubation due to respiratory complications (hypercapnia or sever hypoxemia) (14-17,21-23). In this sense, due to the absence of vascular dissection and the short duration of the procedure, it is very unusual to face with an emergent situation. However, it is more than advisable to have already performed emergency protocols before start NI-VATS program and have qualified staff to control an emergent situation (i.e., NI-VATS major bleeding management) and start conversion from NI-VATS to intubation and GA-VATS (23,26).
About locoregional anesthesia in this procedure, the evidence it is also limited, but currently it seems that control with local anesthesia in the wound and intercostal block is enough to obtain good pain control compared to the use of TEA, without having an impact on early recovery and avoiding the potential risks of the TEA (21). However, the presence of randomized prospective studies with a greater number of patients is still necessary to obtain more solid evidence.
In our experience, preliminary results after performing routine uniportal NI-VATS approach for PSP since 2013 show a convenient improvement of self-center morbidity, finding less sore throat, less hoarseness and early postoperative recovery of patients with and improving in the operative room time. So we encourage different centres to start NI-VATS programs. Nevertheless, future multicentric and prospective randomized trials will be needed to get strong evidence in this topic.
Conclusions
The treatment of PSP using the NI-VATS approach is safe and feasible. Nevertheless, there is a need for a larger number of prospective randomized studies that specifically compare the NI-VATS approach versus GA-VATS for the treatment of PSP.
Surgical technique is not different from GA-VATS procedures, the concern should be focus in locoregional anesthesia, and adapt surgeon’s skills to NI-VATS field (mediastinal movement, diaphragmatic motion, initial cough reflex…).
Locoregional anesthesia through the use of local anesthesia in the wound and intercostal block at the beginning and end of surgery is a safe alternative and shortens the operation room time compared to the use of TEA. Vagal blockade is a fast and safe step that allows to controlling cough reflex.
It is recommended that NI-VATS surgeons have already completed the learning curve for VATS major procedures.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jin-Shing Chen, Ke-Cheng Chen and Mong-Wei Lin) for the series “VATS: Primary Spontaneous Pneumothorax” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2019.09.02). The series “VATS: Primary Spontaneous Pneumothorax” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gupta D, Hansell A, Nichols T, et al. Epidemiology of pneumothorax in England. Thorax 2000;55:666-71. [Crossref] [PubMed]
- Krüger M, Ermitsch M, Uschinsky K, et al. Results of video-assisted thoracoscopic surgery for pneumothorax. Zentralbl Chir 2003;128:645-51. [PubMed]
- Plojoux J, Froudarakis M, Janssens JP, et al. New insights and improved strategies for the management of primary spontaneous pneumothorax. Clin Respir J 2019;13:195-201. [Crossref] [PubMed]
- Tschopp JM, Bintcliffe O, Astoul P, et al. ERS task force statement: diagnosis and treatment of primary spontaneous pneumothorax. Eur Respir J 2015;46:321-35. [Crossref] [PubMed]
- MacDuff A, Arnold A, Harvey JBTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii18-31. [Crossref] [PubMed]
- Vuong NL, Elshafay A, Thao LP, et al. Efficacy of treatments in primary spontaneous pneumothorax: A systematic review and network meta-analysis of randomized clinical trials. Respir Med 2018;137:152-66. [Crossref] [PubMed]
- Pagès PB, Delpy JP, Orsini B, et al. Propensity score analysis comparing videothoracoscopic lobectomy with thoracotomy: a french nationwide study. Ann Thorac Surg 2016;101:1370-8. [Crossref] [PubMed]
- Hwang J, Shin JS, Son JH, et al. Non-intubated thoracoscopic bullectomy under sedation is safe and comfortable in the perioperative period. J Thorac Dis 2018;10:1703-10. [Crossref] [PubMed]
- Medina CR, Camargo Jde J, Felicetti JC, et al. Post-intubation tracheal injury: report of three cases and literature review. J Bras Pneumol 2009;35:809-13. [Crossref] [PubMed]
- Sesma J, Álvarez M, Gálvez C, et al. Diaphragmatic motion during NI-VATS procedure. Asvide 2019;6:270. Available online: http://www.asvide.com/watch/32955
- Kao MC, Lan CH, Huang CJ. Anesthesia for awake video-assisted thoracic surgery. Acta Anaesthesiol Taiwan 2012;50:126-30. [Crossref] [PubMed]
- Galvez C, Bolufer S, Navarro-Martinez J, et al. Non-intubated video-assisted thoracic surgery management of secondary spontaneous pneumothorax. Ann Transl Med 2015;3:104. [PubMed]
- Tusman G, Böhm SH, Sipmann FS, et al. Lung recruitment improves the efficiency of ventilation and gas exchange during one-lung ventilation anesthesia. Anesth Analg 2004;98:1604-9. [Crossref] [PubMed]
- Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg 2011;254:1038-43. [Crossref] [PubMed]
- Tseng YD, Cheng YJ, Hung MH, et al. Nonintubated needlescopic video-assisted thoracic surgery for management of peripheral lung nodules. Ann Thorac Surg 2012;93:1049-54. [Crossref] [PubMed]
- Hung MH, Hsu HH, Chen KC, et al. Nonintubated thoracoscopic anatomical segmentectomy for lung tumors. Ann Thorac Surg 2013;96:1209-15. [Crossref] [PubMed]
- Hung MH, Hsu HH, Chan KC, et al. Non-intubated thoracoscopic surgery using internal intercostal nerve block, vagal block and targeted sedation. Eur J Cardiothorac Surg 2014;46:620-5. [Crossref] [PubMed]
- Baird AG, Lawrence JR. Guidelines: is bigger better? A review of SIGN guidelines. BMJ Open 2014;4:e004278 [Crossref] [PubMed]
- Li S, Cui F, Liu F, et al. Nonintubated uniportal video-assisted thoracoscopic surgery for primary spontaneous pneumothorax. Chin J Cancer Res 2015;27:197-202. [PubMed]
- Guo Z, Yin W, Zhang X, et al. Primary spontaneous pneumothorax: simultaneous treatment by bilateral non-intubated videothoracoscopy. Interact Cardiovasc Thorac Surg 2016;23:196-201. [Crossref] [PubMed]
- Guo Z, Yin W, Wang W, et al. Spontaneous ventilation anaesthesia: total intravenous anaesthesia with local anaesthesia or thoracic epidural anaesthesia for thoracoscopic bullectomy. Eur J Cardiothorac Surg 2016;50:927-32. [Crossref] [PubMed]
- Kiss G, Castillo M. Nonintubated anesthesia in thoracic surgery: general issues. Ann Transl Med 2015;3:110. [PubMed]
- Galvez C, Navarro-Martinez J, Bolufer S, et al. Nonintubated uniportal VATS pulmonary anatomical resections. J Vis Surg 2017;3:120. [Crossref] [PubMed]
- Sesma J, Álvarez M, Gálvez C, et al. Vagal block in NI-VATS procedures. Right paratracheal vagal block and left aortopulmonary vagal block. Asvide 2019;6:271. Available online: http://www.asvide.com/watch/32956
- Sesma J, Álvarez M, Gálvez C, et al. Cough reflex before vagal block in NI-VATS approach. Asvide 2019;6:272. Available online: http://www.asvide.com/watch/32957
- Navarro-Martínez J, Gálvez C, Rivera-Cogollos MJ, et al. Intraoperative crisis resource management during a non-intubated video-assisted thoracoscopic surgery. Ann Transl Med 2015;3:111. [PubMed]
- Sesma J, Álvarez M, Gálvez C, et al. Intercostal nerve block. Asvide 2019;6:273. Available online: http://www.asvide.com/watch/32958
- Sesma J, Álvarez M, Gálvez C, et al. NI-VATS bullectomy for PSP with apical bulla. Asvide 2019;6:274. Available online: http://www.asvide.com/watch/32959
- Sesma J, Álvarez M, Gálvez C, et al. NI-VATS bullectomy for PSP with giant bulla. Asvide 2019;6:275. Available online: http://www.asvide.com/watch/32960
- Sesma J, Álvarez M, Gálvez C, et al. NI-VATS talc pleurodesis for PSP treatment. Asvide 2019;6:276. Available online: http://www.asvide.com/watch/32961
Cite this article as: Sesma J, Álvarez M, Gálvez C, Bolufer S, Lirio F, Mafé JJ, Del Campo JM, Maroto S, Navarro-Martínez J, Rivera MJ, Galiana M, Cerezal J. Non-intubated VATS for the management in primary spontaneous pneumothorax. Video-assist Thorac Surg 2019;4:23.