
Effects of picibanil as sclerosing agent in primary spontaneous pneumothorax patient after thoracoscopic procedures
Introduction
Primary spontaneous pneumothorax (PSP) is defined as the presence of air into the pleural space without evident thoracic trauma or underlying pulmonary disease, which most commonly occurs in young, tall, lean males (1,2). For patients suffered from spontaneous pneumothorax, there are three clinical issues to solve. The first issue is pneumothorax caused symptoms, such as chest pain, chest tightness, dyspnea, or the worst desaturation with respiratory failure, which could be relieved by tube thoracostomy, pain killer and oxygen supply (3). The second clinical problem is associated with poor healing of lung parenchyma and persistent air-leakage after tube thoracostomy or video-assisted thoracic surgery (VATS) (4). And the last and the most important clinical issue for patient suffered from pneumothorax is recurrence of pneumothorax. Without intervention, recurrence rate was as high as 49% in 1 year (5,6). The surgical indication for PSP had been proposed as recurrence of pneumothorax, prolonged air-leakage, complicated with hemothorax, bilateral pneumothorax and patients with specific risks of atmosphere change, such as pilots, flight attendants or deep-sea divers (3,7,8). VATS is regarded as superior to thoracotomy approach in terms of reduced pain, less invasive, shorter days of hospital stay and fewer atelectasis following the procedures (9). Unfortunately, VATS bullectomy alone was associated with higher recurrence rate between 10% and 24% and recurrence rate of open thoracotomy was around 3% (4,10,11). Although, in most practice guidelines, chemical pleurodesis is regarded as the salvage for patients who would not tolerate surgical intervention to treat pneumothorax (3,12). When VATS bullectomy combined with pleurectomy, some kind of pleurodesis, either mechanical abrasion or chemical pleurodesis, the risk of recurrence was reduced to 1–6% (13-16).
There have been a lot of sclerosants used for chemical pleurodesis, to achieve symphysis between the visceral and parietal pleura to prevent recurrent pleural effusion or recurrent pneumothorax, including talc, tetracycline and its derivatives, minocycline, bleomycin, autologous blood patch, iodopovidone, picibanil (OK-432), and silver nitrate (17). OK-432, a lyophilized preparation of heat-killed Su-strain of type 3, group A Streptococcus pyogenes, is a chemical irritant that has been used in sclerotherapy for postoperative air-leakage, intractable pneumothorax and malignant pleural effusions (17-20). In this study, we report our clinical experience of using OK-432 for additional chemical pleurodesis after patients received VATS bullectomy for PSP.
Methods
Study design and patients
The objective of this retrospective study was to evaluate the safety and efficacy of OK-432 as sclerosing agents in patients after VATS for PSP. We cautiously review the medical records of these patients with pneumothorax, who received VATS bullectomy in National Taiwan University Hospital between April 1994 and May 2014. Patients, who was aged over 50 years old or had underlying pulmonary diseases, were excluded from current study to avoid patients with secondary pneumothorax. This study was reviewed and approved by the Research Ethics Committee of National Taiwan University Hospital (approval number 201202011RIC).
VATS procedures
Under general anesthesia and one-lung ventilation, patient received needlescopic or conventional VATS for bullectomy with or without mechanical pleurodesis. There was no difference between needlescopic and conventional VATS in methods of anesthesia, patient preparations, procedures of operations and post-operative recurrence rate (21,22).
Under thoracoscopic surveillance, adhesions between lung surface and chest wall were freed using electrocautery directly. When blebs, most at apex of upper lobes, were identified, they were removed by wedge resection by using an endoscopic stapler. Blind stapling at apical area, the most suspicious area, was performed, if no bleb or active air-leakage could be identified. Pleural abrasion method was chosen for mechanical pleurodesis (23). Lung re-inflation method was applied to check air-leakage, when normal saline was instilled into pleural space. One chest tube (28 Fr) or pigtail (8 Fr) was inserted through the incision wounds to place at the apex area of upper lobe.
After the VATS bullectomy and pleurodesis, extubation was performed in the operating room and then the patient was observed for one to two hours in the recovery room. Chest roentgenogram was scheduled on the next coming morning. The thoracic drainage system was connected to a low-pressure suction (−10 cmH2O), if the lung was not fully expanded on chest roentgenogram.
Chemical pleurodesis using OK-432
When postoperative air-leakage occurred or instilled for prophylactic use to reduce recurrence, OK-432 was chosen for chemical pleurodesis to achieve the adhesion between the two layers of pleura. The procedure of OK-432 pleurodesis was as following, the first step was instilled 20 mL of 2% lidocaine hydrochloride (400 mg) into the pleural space via thoracic drainage tube, and then instilled the 20 mL of normal saline solution containing 5–10 Klinische Einheit (KE) of OK-432 (1 KE equals to 0.1 mg of dried cocci; Chugai Pharmaceutical Co., Tokyo, Japan) (17). The drainage tube between the thoracic drainage tube (chest tube or pigtail) and chest bottle was raised 40 to 60 cm above the patient to indwell the sclerosing agent and allow air to pass through at the same time. Patients were ordered to change positions (left-side decubitus, supine or right-side decubitus position) every 30 minutes, in order to make the sclerosing agent contacting all over the whole pleural surfaces. Associated side effects and complaints of pleurodesis were documented. The rubber tube was lowered 2 hours later to drainage the effusion out.
The postoperative pain was assessed 3 times per day and the intensity of pain was recorded by the visual analogue scale (VAS; zero indicated no pain and ten indicated the most severe, intractable pain). If the post-operative pain was not relieved by oral analgesics, and VAS was greater than seven, meperidine hydrochloride (Demerol®, 50mg/ampule) would be prescribed for pain control. The thoracic drainage tube would be removed when the lung was fully expanded and no more air-leakage was noted in the past 24 hours period.
All adverse events and complains were documented. Fever was defined as body core temperature ≥38 °C. Loculated effusion was routinely checked on chest roentgenogram (CXR) and sonography after pleurodesis.
Follow-up and pulmonary function analysis
After discharge from the hospital, all patients were scheduled to follow at the outpatient clinics, where CXR was arranged to check if recurrent pneumothorax occurred and pulmonary function tests were also performed. The latest patient followed up in May 2018. Spirometer (Microspiro HI-298; Chest Corporation, Tokyo, Japan) was used to estimate forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1.0). Three acceptable forced expiratory maneuvers were recorded, and analyzed.
Data collection and analysis
The patient demographic data and related surgical results including, operative findings, dosed and times of pleurodesis, post-operative pain scale (VAS) and doses of meperidine, complications, duration of chest tube drainage retained, and length of hospital stay were collected through retrospective chart review. Continuous variables such as age or height were expressed as mean ± standard deviation. Categorical variables such as sex or smoking status were presented by frequency (%).
Results
Between April 1994 and May 2014, the total number 1,695 patients with PSP were treated by VATS. Routine postoperative follow-up and data collection for check postoperative events and recurrence of pneumothorax were documented till 2018 May. This study enrolled 89 patients, who received chemical pleurodesis with OK-432 after VATS procedure. The result of the patient demographics, pre-operative characteristics, indications for surgery, and intra-operative finding are summarized in Table 1. Patients consisted of 66 male and 23 female, with a median age of 23 years. The mean body mass index of all patients was 18.7 kg/m2. About 1 of 5 patients had smoking habit of cigarettes. The incidence of left-side pneumothorax was 52.8%, included three patients had bilateral pneumothorax. The indication for VATS bullectomy was as following recurrent pneumothorax (67.4%), persistent air-leakage (21.3%), bilateral concurrent pneumothorax (3.4%), hemopneumothorax (2.2%) and uncomplicated first episode of pneumothorax (5.6%). On thoracoscopy, four patients had no endoscopic abnormalities (Vanderschueren’s stage 1), 23 patients had pleuropulmonary adhesions (Vanderschueren’s stage 2), 48 patients had blebs or bullae <2 cm in greatest diameter (Vanderschueren’s stage 3), and 14 patients had bullae >2 cm in greatest diameter.
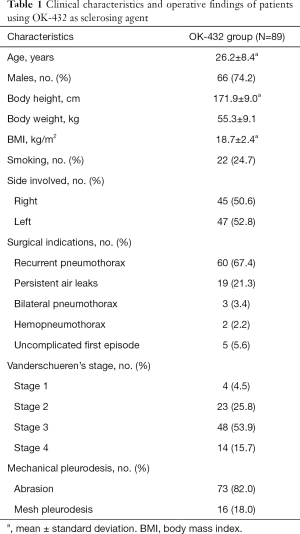
Full table
The mean dose of OK-432 instillation was 7.6±4.2 KE. The clinical characteristics and post-operative results of the patients are summarized in Table 2. Wound pain was the most common complaint after VATS procedure and the severity was decreased gradually. Fever was the most frequent complication after chemical pleurodesis with OK-432, and the following was loculated effusion. Fever was resolved by took acetaminophen 500 mg. One patient developed loculated effusions on CXR that resolved gradually without any invasive interventions. Thirteen patients complained severe pain that required immediate meperidine intramuscular injection and the dose of meperidine were 59.9±79.0 mg.
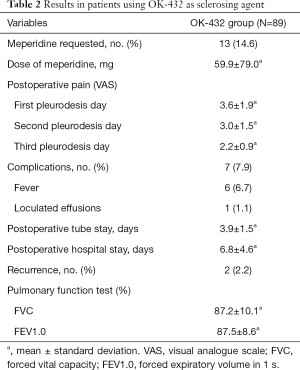
Full table
The durations of chest tube drainage and the post-operative stay hospitalization were 3.9±1.5 and 6.8±4.6 days separately. Until the latest post-operative follow-up in May 2018, only two patients (2.2%) suffered from ipsilateral recurrent pneumothorax. The pneumothorax resolved spontaneously without tube thoracostomy or surgical intervention. Pulmonary function was measured by Spirometer to evaluate the influence of chemical pleurodesis with Ok-432 (Table 2).
Discussion
This study demonstrates that chemical pleurodesis with OK-432 following VATS procedure provides a safe, and effective treatment for PSP.
The therapeutic aims of pneumothorax focused on evacuating air from the pleural space (symptoms relief), ceasing air leakage (parenchyma healing), and preventing recurrences (3). To prevent recurrence of pneumothorax is the most important therapeutic challenge in the management of PSP. Many treatments are proposed to reduce recurrence of pneumothorax included simple aspiration, tube thoracostomy with chemical pleurodesis or pulmonary resection by open thoracotomy or VATS with mechanical pleurodesis (24). The options of management may depend on the severity of clinical manifestations, the medical and social background of patients, availability of skilled physicians and facility of medical center.
Chemical pleurodesis, played a crucial role of stopping air-leak or preventing recurrent pneumothorax, which could be instilled through the thoracic drainage tube, medical thoracoscopy, or during the VATS procedure. Pleurodesis means to achieve symphysis between parietal and visceral pleura and to prevent relapse of pneumothorax (8). There have been a lot of sclerosing agents used for chemical pleurodesis, to prevent recurrent pleural effusion or pneumothorax, including talc, tetracycline, minocycline, bleomycin, autologous blood patch, iodopovidone, OK-432, silver nitrate, and quinacrine (17). Light et al. conducted a prospective randomized trial and showed that intrapleural instillation of tetracycline via thoracostomy tube could diminish incidence of recurrence of PSP (25).
In general practice, surgical intervention was indicated when patients faced with recurrent or complicated spontaneous pneumothorax. Chemical pleurodesis could be an adjunct after drainage or surgery. Although, VATS is regarded as better than thoracotomy approach in terms of pain relief, minimal invasive, shorter days of hospital stay and less pulmonary complications (9). The higher recurrence rate of VATS bullectomy was an unfavorable concern. The recurrence rate of VATS bullectomy alone and open thoracotomy was 10% to 24% and 3% separately (4,10,11). Additional pleurodesis following VATS bullectomy could effectively reduce the risk of recurrence to 1–6% (13-16). There are numerous benefits of additional chemical pleurodesis after VATS for PSP. In addition to prevent recurrence of pneumothorax, it may shorten the durations of post-operative air-leakage and hospital stay (17,26).
In this study, following VATs procedure, additional pleurodesis with OK-432, a product of heat-killed Streptococcus pyogenes, was used to prevent recurrence. There were several study of OK-432 focused on ceasing post-operative air-leakage, diminishing recurrence of pneumothorax, malignant pleural effusion, and the safety in patients with interstitial pneumonia. These studies were summarized in Table 3. In this study, our data demonstrated that pleurodesis with OK-432 is also safe and effective with low recurrence rate (2.2%) after VATS for PSP. In our hospital, OK-432 was initially used as a salvage treatment for prolonged, and intractable air-leakage after chemical pleurodesis with minocycline. In recent years, OK-432 was used as the primary treatment for additional chemical pleurodesis after VATS procedure.
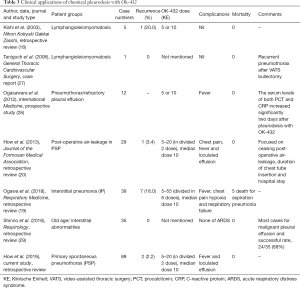
Full table
Fever was the most common compliant associated with OK-432 pleurodesis (6.7%). In clinical practice, chills and fever usually developed a few hours after OK-432 instillation, but resolved within one day after took acetaminophen. The significantly increased serum level of procalcitonin (PCT) or C-reactive protein (CRP) were also reported by Ogasawara et al. (27). Another concern with instillation of sclerosing agents into pleural space for chemical pleurodesis is empyema. In our study population, only one patient with loculated effusions was found by CXR. Our results suggest that pleurodesis by OK-432 associated with low incidence of pleural infection.
The more intense pleurodesis may result in more restrictive pulmonary dysfunction. The results of pulmonary function tests after pleurodesis did not showed any restrictive or obstructive pattern.
There are several limitations of present study. At the first, it is a retrospective chart review study of a group of patients treated by several surgeons during a 20-year period. There was no standardized treatment policy for all patients with PSP and treatments were modified over time through. We believe that a prospective study with precise definition of variables and standardized treatment protocol will be helpful in validating our results.
In conclusion, this study demonstrates that using Ok-432 as additional chemical pleurodesis for patients with PSP after VATS procedures is safe and effective with low recurrence rate.
Acknowledgments
Funding: This work was supported by a research grant from the Far Eastern Memorial Hospital and National Taiwan University Hospital Joint Research Program.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Video-Assisted Thoracic Surgery for the series “VATS: Primary Spontaneous Pneumothorax”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2019.07.02). The series “VATS: Primary Spontaneous Pneumothorax” was commissioned by the editorial office without any funding or sponsorship. JSC served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from May 2018 to Apr 2020. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the Research Ethics Committee of National Taiwan University Hospital (approval number 201202011RIC). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lichter I, Gwynne JF. Spontaneous pneumothorax in young subjects. A clinical and pathological study. Thorax 1971;26:409-17. [Crossref] [PubMed]
- Sahn SA, Heffner JE. Spontaneous pneumothorax. N Engl J Med 2000;342:868-74. [Crossref] [PubMed]
- MacDuff A, Arnold A, Harvey J. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii18-31. [Crossref] [PubMed]
- Muramatsu T, Nishii T, Takeshita S, et al. Preventing recurrence of spontaneous pneumothorax after thoracoscopic surgery: a review of recent results. Surg Today 2010;40:696-9. [Crossref] [PubMed]
- Chen JS, Chan WK, Tsai KT, et al. Simple aspiration and drainage and intrapleural minocycline pleurodesis versus simple aspiration and drainage for the initial treatment of primary spontaneous pneumothorax: an open-label, parallel-group, prospective, randomised, controlled trial. Lancet 2013;381:1277-82. [Crossref] [PubMed]
- Noppen M, Alexander P, Driesen P, et al. Manual aspiration versus chest tube drainage in first episodes of primary spontaneous pneumothorax: a multicenter, prospective, randomized pilot study. Am J Respir Crit Care Med 2002;165:1240-4. [Crossref] [PubMed]
- Dugan KC, Laxmanan B, Murgu S, et al. Management of Persistent Air Leaks. Chest 2017;152:417-23. [Crossref] [PubMed]
- Tschopp JM, Rami-Porta R, Noppen M, et al. Management of spontaneous pneumothorax: state of the art. Eur Respir J 2006;28:637-50. [Crossref] [PubMed]
- Sedrakyan A, van der Meulen J, Lewsey J, et al. Video assisted thoracic surgery for treatment of pneumothorax and lung resections: systematic review of randomised clinical trials. BMJ 2004;329:1008. [Crossref] [PubMed]
- Horio H, Nomori H, Kobayashi R, et al. Impact of additional pleurodesis in video-assisted thoracoscopic bullectomy for primary spontaneous pneumothorax. Surg Endosc 2002;16:630-4. [Crossref] [PubMed]
- Nakanishi K. Long-term effect of a thoracoscopic stapled bullectomy alone for preventing the recurrence of primary spontaneous pneumothorax. Surg Today 2009;39:553-7. [Crossref] [PubMed]
- Tschopp JM, Bintcliffe O, Astoul P, et al. ERS task force statement: diagnosis and treatment of primary spontaneous pneumothorax. Eur Respir J 2015;46:321-35. [Crossref] [PubMed]
- Imperatori A, Rotolo N, Spagnoletti M, et al. Risk factors for postoperative recurrence of spontaneous pneumothorax treated by video-assisted thoracoscopic surgerydagger. Interact Cardiovasc Thorac Surg 2015;20:647-51; discussion 651-2. [Crossref] [PubMed]
- Neudecker J, Malzahn U, Heuschmann P, et al. Pulmonary wedge resection plus parietal pleurectomy (WRPP) versus parietal pleurectomy (PP) for the treatment of recurrent primary pneumothorax (WOPP trial): study protocol for a randomized controlled trial. Trials 2015;16:540. [Crossref] [PubMed]
- Mithiran H, Leow L, Ong K, et al. Video-Assisted Thoracic Surgery (VATS) Talc Pleurodesis Versus Pleurectomy for Primary Spontaneous Pneumothorax: A Large Single-Centre Study with No Conversion. World J Surg 2019;43:2099-105. [Crossref] [PubMed]
- Ling ZG, Wu YB, Ming MY, et al. The effect of pleural abrasion on the treatment of primary spontaneous pneumothorax: a systematic review of randomized controlled trials. PloS One 2015;10:e0127857 [Crossref] [PubMed]
- How CH, Tsai TM, Kuo SW, et al. Chemical pleurodesis for prolonged postoperative air leak in primary spontaneous pneumothorax. J Formos Med Assoc 2014;113:284-90. [Crossref] [PubMed]
- Kishi K, Homma S, Sakamoto S, et al. High efficacy of pleurodesis using OK-432 for controlling intractable pneumothorax associated with pulmonary lymphangioleiomyomatosis. Nihon Kokyuki Gakkai Zasshi 2003;41:704-7. [PubMed]
- Ogawa K, Takahashi Y, Murase K, et al. OK-432 pleurodesis for the treatment of pneumothorax in patients with interstitial pneumonia. Respir Investig 2018;56:410-7. [Crossref] [PubMed]
- How CH, Hsu HH, Chen JS. Chemical pleurodesis for spontaneous pneumothorax. J Formos Med Assoc 2013;112:749-55. [Crossref] [PubMed]
- Chen JS, Hsu HH, Kuo SW, et al. Needlescopic versus conventional video-assisted thoracic surgery for primary spontaneous pneumothorax: a comparative study. Ann Thorac Surg 2003;75:1080-5. [Crossref] [PubMed]
- Chang YC, Chen CW, Huang SH, et al. Modified needlescopic video-assisted thoracic surgery for primary spontaneous pneumothorax: the long-term effects of apical pleurectomy versus pleural abrasion. Surg Endosc 2006;20:757-62. [Crossref] [PubMed]
- Chen JS, Hsu HH, Huang PM, et al. Thoracoscopic pleurodesis for primary spontaneous pneumothorax with high recurrence risk: a prospective randomized trial. Ann Surg 2012;255:440-5. [Crossref] [PubMed]
- Vuong NL, Elshafay A, Thao LP, et al. Efficacy of treatments in primary spontaneous pneumothorax: A systematic review and network meta-analysis of randomized clinical trials. Respir Med 2018;137:152-66. [Crossref] [PubMed]
- Light RW, O'Hara VS, Moritz TE, et al. Intrapleural tetracycline for the prevention of recurrent spontaneous pneumothorax. Results of a Department of Veterans Affairs cooperative study. JAMA 1990;264:2224-30. [Crossref] [PubMed]
- Chen JS, Hsu HH, Chen RJ, et al. Additional minocycline pleurodesis after thoracoscopic surgery for primary spontaneous pneumothorax. Am J Respir Crit Care Med 2006;173:548-54. [Crossref] [PubMed]
- Taniguchi Y, Haruki T, Fujioka S, et al. Pulmonary lymphangioleiomyomatosis with concomitant tuberous sclerosis complex diagnosed by video-assisted thoracoscopic surgery. Gen Thorac Cardiovasc Surg 2008;56:81-4. [Crossref] [PubMed]
- Ogasawara T, Umezawa H, Kato S, et al. Intrathoracic administration of OK-432 elevates the serum procalcitonin levels. Intern Med 2012;51:2727-31. [Crossref] [PubMed]
- Shinno Y, Kage H, Chino H, et al. Old age and underlying interstitial abnormalities are risk factors for development of ARDS after pleurodesis using limited amount of large particle size talc. Respirology 2018;23:55-9. [Crossref] [PubMed]
Cite this article as: How CH, Hsu HH, Chen JS. Effects of picibanil as sclerosing agent in primary spontaneous pneumothorax patient after thoracoscopic procedures. Video-assist Thorac Surg 2019;4:16.


