Video-assisted thoracic surgery lobectomy beyond the learning curve
In the last decade an increasing, more and more widespread use of video-assisted thoracic surgery (VATS) anatomical lung resections has become a standard primary operative procedure in many thoracic units worldwide, especially in high volume centers. VATS surgery was proved to be superior to a conventional open thoracotomy approach in regard to postoperative enhanced recovery and at least equivalent mid to long-term oncological outcome. This issue could be evidenced and demonstrated in many recent studies (1-4).
It is generally accepted that a learning curve of approximately 30 to 50 individual operative VATS-lobectomy cases is necessary to gain sufficient confidence with a thoracoscopic approach to lobectomy in order to include also higher than stage I tumors in the routine-operative schedule subsequently.
The retrospective review article by Amore and coauthors (5), analyzing 571 consecutive patients undergoing VATS lobectomy in a single institution between 2011 and 2017, shows very well how the reasons for conversion to open thoracotomy changed with increasing experience after completion of a learning curve of 50 operative cases. During the learning curve-period the most frequent reasons for conversion were an incomplete fissure, enlarged mediastinal or hilar lymphnodes, intraoperative bleeding due to vessel injury and pleural adhesions, resulting in a conversion rate of 18% (9 out of 50). In the period after the learning curve the major causes for conversion were intraoperative bleeding due to vessel injury mainly to the pulmonary artery, followed by malignant and/or benign, calcified hilar lymphadenopathy, vascular adventitial fibrosis and incomplete fissures. The rate of conversion decreased to 5.9% in the later period, although also more complex cases were included.
Some years ago, our group (6) could show in a multivariate logistic regression analysis (P=0.013) that the only significant risk factor for conversion was administration of neoadjuvant chemotherapeutic treatment leading to a more than 4-fold risk for conversion to open thoracotomy. Furthermore, tumor size was also an independent risk factor for conversion (P=0.04) in our series of 232 consecutive patients from 2009 to 2012, especially in the first half of the study period. Here we also noted a trend to bleeding as a major cause for conversion, whereas in the later period there was a trend to conversion mainly because of oncological/technical reasons, like massive pleural adhesions. Neoadjuvant treatment and tumor size predominantly lead to an overall conversion rate of 6.5% (15 patients) in our study. Nevertheless, conversion to open thoracotomy did not result in a higher overall rate of postoperative complications, longer chest drain duration or in-hospital mortality, but significantly increased length of hospital stay in these patients, i.e., median 11 vs. 9 days in VATS patients (P=0.028).
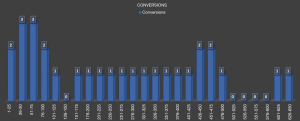
Similarly, our findings could also be confirmed to date and are outlined in Figure 1. The overall conversion rate in 650 consecutive patients who underwent VATS anatomical lung resections in our center is actually 4.9%. Advanced and more complex cases like VATS broncho/angioplatic procedures, bilobectomies, pneumonectomies and segmentectomies are included.
With increasing experience and confidence in VATS surgery we were also able to improve ability for successful management of some intraoperative complications by minimal invasive “trouble-shooting”, particularly bleeding from pulmonary vessels or consistent bronchial air-leaks (7). Minimal invasive control of an eventual intraoperative complication also contributed to a decreasing rate of conversions. Currently, in most centers performing a high volume VATS program conversion rate is reported to range between 5% and 6%. The authors of the discussed article also confirm this by their experience. Although incomplete fissures and malignant or benign/calcified enlarged hilar lymphnodes sometimes represent a consistent problem in VATS lobectomy we could not outline this as an independent significant risk factor for conversion in our series. It would be interesting to know, if the authors noted a higher prevalence of previous and/or concomitant chronic pulmonary disorders in their patients, for example history of lung tuberculosis, silicosis or chronic emphysema. Furthermore, there is no note on the rate of neoadjuvant treated patients, which could also represent a significant part of their reported cases converted to thoracotomy. Nevertheless, In our study we could not evidence that VATS conversion rate was statistically influenced by patient age, nodal stage (pN0 vs. pN+), body mass index, presence of chronic obstructive pulmonary disease, lung function (FEV1) or benign lymphnode disease (6).
During our experience in minimal invasive anatomical lung resections of almost 10 years, when performing a VATS lobectomy we learned that it is of paramount importance to be prepared for critical intraoperative situations resulting from various potentially dangerous risk factors for conversion as referred above. Planned strategies to handle intraoperative complications and interdisciplinar communication of these with anesthesiological and operative teams are the key for an uneventful intra- and perioperative management. As a primary issue, patient safety comes first and conversion to thoracotomy—open surgery should not be considered as a failure.
In accordance with the authors of the paper discussed, we would also wish to highlight the importance of identifying clear preoperative risk factors when performing a minimal invasive, anatomical lung resection in order to further reduce rates of an unexpected conversion to thoracotomy in an eventual emergency setting. On the other hand, we also point out that “no conversion is absolutely not to be considered equivalent to no complication”; concomitantly, intraoperative trouble-shooting by intraoperative minimal invasive complication management should be implemented particularly in those centers with a growing and robust experience in VATS anatomical lung resections.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Federico Raveglia, MD (Department of Thoracic Surgery, ASST—Santi Paolo e Carlo, University of Milan Medical School, Milan, Italy).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2018.07.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Cao C, Manganas C, Ang SC, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small cell lung cancer: a meta-analysis of propensity score-matched patients. Interact Cardiovasc Thorac Surg 2013;16:244-9. [Crossref] [PubMed]
- Nwogu CE, D'Cunha J, Pang H, et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg 2015;99:399-405. [Crossref] [PubMed]
- Wu CF, Fernandez R, de la Torre M, et al. Mid-term survival outcome of single-port video-assisted thoracoscopic anatomical lung resection: a two-centre experience. Eur J Cardiothorac Surg 2018;54:252-9. [Crossref] [PubMed]
- Amore D, Di Natale D, Scaramuzzi R, et al. Reasons for conversion during VATS lobectomy: what happens with increased experience. J Vis Surg 2018;4:53. [Crossref] [PubMed]
- Augustin F, Maier HT, Weissenbacher A, et al. Causes, predictors and consequences of conversion from VATS to open lung lobectomy. Surg Endosc 2016;30:2415-21. [Crossref] [PubMed]
- Lucciarini P, Augustin F, Maier HT, et al. Intraoperative complications during VATS lobectomies from conversion to minimally-invasive "trouble-shooting". J Vis Surg 2018;4:28. [Crossref] [PubMed]
Cite this article as: Lucciarini P, Augustin F, Maier H, Schmid T. Video-assisted thoracic surgery lobectomy beyond the learning curve. Video-assist Thorac Surg 2018;3:30.