
Video-assisted thoracic surgery double sleeve lobectomy for non-small cell lung cancer: a report of seven cases
Introduction
Video-assisted thoracic surgery (VATS) lobectomy has become routine in many centers for the treatment of early stage non-small cell lung cancer (NSCLC). Since major advances have been achieved in surgical skills and instruments, sporadic cases of complete VATS bronchial sleeve lobectomy, once considered as a contraindication for VATS pulmonary resection, have been successfully performed in several experienced centers during the past decade (1-5). We started to perform VATS bronchial sleeve lobectomy since December 2010 (6,7). With accumulative experiences, we attempted to explore the feasibility of VATS bronchovascular double sleeve lobectomy. This report contributes the techniques and outcomes of this challenging surgical procedure in our center.
Methods
From May 2012 to December 2016, we performed seven cases of VATS bronchovascular double sleeve lobectomy at the Department of Thoracic Surgery, West China Hospital, Sichuan University. Clinical records of these patients were retrieved from the Western China Lung Cancer Database. All the patients with lung cancer, who underwent surgery in our department, were enrolled in this database since late 2005. Clinical data of these patients were collected prospectively. The institutional review board (IRB) of West China Hospital approved the use of these clinical data (No. 2016-98).
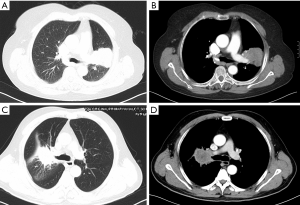
There were one female patient and six male patients, and five of them were smokers. The median age of these patients was 68.5 (ranging from 61 to 72 years old). All the patients were diagnosed as NSCLC by bronchoscopy before surgery, including two cases of adenocarcinoma and five cases of squamous cell carcinoma. One patient had clinical N2 disease and received two cycles of platinum-based induction chemotherapy. Detailed information of these patients was listed in Table 1. Preoperative clinical staging was based on contrast chest and upper abdominal computed tomography (CT) scan, brain contrast magnetic resonance imaging (MRI) scan, and single photon emission computed tomography (SPECT) scan of the bone. Representative CT scans of the tumor were shown in Figure 1. Meanwhile, general assessment of these patients also included routine laboratory tests, electrocardiograms, pulmonary function tests, and echocardiography. Alternatives for the treatment were fully informed to these patients. Informed consent of the operation was obtained from all the patients.

Full table
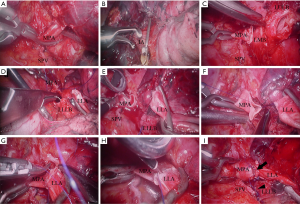
Four patients underwent VATS left upper double sleeve lobectomy, and the other three underwent VATS right upper double sleeve lobectomy. The operation was carried out under general anesthesia with double-lumen endobronchial intubation. The patient was placed in the lateral decubitus position during surgery. Preliminary procedure of the operation has been described previously (8). During our first attempt to perform the VATS left upper double sleeve lobectomy, four ports were made, including a thoracoscopic port in the eighth intercostal space (ICS) on the mid-axillary line, an assistant port in the seventh ICS on the posterior axillary line, and the other two ports on the anterior axillary line in the third and sixth ICS, respectively. For the other cases, we moved the thoracoscopic port to the sixth or seventh ICS on the mid-axillary line to get better view for bronchovascular reconstruction. One utility port was made in the third ICS on the anterior axillary line, with only one assistant port in the seventh ICS for the left side, while in the eighth ICS for the right side, both on the posterior axillary line.
After a thorough exploration of the chest, the main pulmonary artery and both pulmonary veins were mobilized first. We designed the “hollow out” process for this challenging operation. The hilum and mediastinal lymph nodes were removed to “hollow out” the vital hilum structures. Interlobar artery was then mobilized after the pulmonary fissure was dissected. The main pulmonary artery and interlobar artery were blocked using two releasable atraumatic endoscopic Bulldog Clamps (Aesculap, Inc., Center Valley, PA, USA). Superior pulmonary vein, main pulmonary artery, and bronchus were then transected one by one, followed by bronchial and pulmonary artery reconstruction. Both of the bronchial and vascular margins were confirmed negative by frozen section.
The “two-needle-holder suturing technique” was developed for bronchovascular reconstruction. Two needle holders (one endoscopic needle holder and one long handle needle holder) were introduced through different ports simultaneously during the suture. One needle holder was used to perform the suture with the assistance of the other one, and vise versa. Exchange of the instruments through different ports was avoided during the anastomosis with this technique. Bronchial anastomosis was accomplished using running suture with 3-0 Prolene stiches with two needles. The anastomotic stoma was checked for air leak with water. The pulmonary artery was then reconstructed using 5-0 running Prolene stiches with two needles (Figure 2). A piece of polyglycolic acid felt (Neoveil®, Igaki Medical Planning Co. Ltd., Kyoto, Japan) was applied to wrap the bronchial anastomosis. Chest tube was placed through the thoracoscopic port before closing the incisions.
Postoperative management of the patients was mostly similar with our routine sleeve lobectomy. Intravenous patient-controlled analgesia device was used after the surgery for pain control. FOB was performed to clear airway secretions on postoperative day 1. Low molecular heparin was administered through subcutaneous injection during the first week after the surgery. The patients were transferred to a medical oncologist for chemotherapy after the first 1 month follow-up.
Results
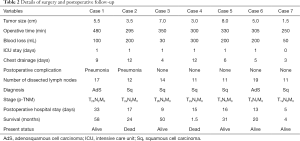
The operations were uneventful and accomplished within 250 to 480 min (median, 318 min). There were no conversions to thoracotomy. Significant pleural adhesion was identified during the operation of the second case and was overcome through thoracoscopy. The blood loss was 30 to 200 mL (median, 200mL), and no blood transfusion was needed. The average number of the dissected lymph nodes was 13 (range, 11–19). Detailed information about the operations was listed in Table 2. Six patients were transferred to the intensive care unit (ICU) with bronchial intubation after surgery, except the last case. They were extubated when recovered well from anesthesia. None of the patients needed reintubation or mechanical ventilation after that time. They were transferred to the general ward on the second day after surgery.
Full table
Prolonged air leak (>5 days) was observed in three patients. Two patients developed pneumonia after surgery with no mortalities. The median postoperative hospital stay was 15.5 days (range: 5–33 days). Histological diagnoses of these patients included two cases of adenosquamous cell carcinoma and five cases of squamous cell carcinoma. Pathological examination of the surgical margins confirmed complete resection for every patient. Three patients had N2 lymph node metastasis, including two cases of oligo-N2 disease. The reconstructed bronchus and pulmonary artery continued to function well during postoperative follow-up. One patient died of hemoptysis 50 days after surgery, and one died of metastatic lung cancer 2 years after surgery. The other five patients were alive without local recurrence at 4–58 months of follow-up.
Discussion
VATS is now widely accepted as a reasonable approach for the treatment of early stage NSCLC. Compared with lobectomy performed through thoracotomy, VATS lobectomy offers obvious benefits to the patients and the evidence is increasing year by year. Advances in surgical techniques of mini-invasive lung surgery have led to a less traumatic approach for numerous types of thoracic operations and even complex procedures, such as bronchial or arterial plasty (1-7,9,10). However, hilar tumors invading both the bronchus and pulmonary artery are still considered as contraindication for VATS. Bronchovascular double sleeve lobectomy is an alternative procedure to pneumonectomy if a complete resection can be achieved, to preserve more of the pulmonary parenchyma. The operation itself is full of challenges, including a relatively high risk of postoperative morbidity and mortality. For this operation, conventional surgical approach is the posterolateral thoracotomy.
Based on accumulative experiences of VATS bronchial sleeve lobectomy (6,7) and angiorrhaphy (11), we carefully attempted to perform the first case of bronchovascular double sleeve lobectomy through the thoracoscopic approach worldwide (8). The detailed surgical procedure have been reported previously (8). Since then, we continued to improve the surgical techniques for this tough procedure. After our first report of the VATS double sleeve lobectomy, several experienced teams also tried to perform this challenging procedure with 3 or 4 ports, even the uniportal approach (12-14).
In our opinion, major concerns for the VATS approach to bronchial sleeve lobectomy or bronchovascular double sleeve lobectomy are represented as tumors limited to the orifice of the lobar bronchus, skilled endoscopic suturing skills, proper equipment, confidence of troubleshooting vascular injury during VATS lung surgery, and suitable approaches of the surgical procedure. The seven patients we operated on were all suffering from centrally located lesions with tumors limited to the upper-lobar orifice.
Sufficient access to the bronchus and pulmonary artery is quite important to assure the safety and reliability in controlling the artery, as well as to ensure performance of the anastomosis. For this reason, distribution of the surgical ports is essential to ensure a successful operation. Based on the experience of previous thoracoscopic left upper bronchial sleeve lobectomy (6), four ports were made during the operation of the first patient. We improved the incisions for the next six patients and three ports were made. The fourth ICS offered better access to perform left bronchial and arterial reconstruction. On the right side, we tried the third ICS as the utility incision and found it was more convenient to perform the anastomosis. According to previous practice, we believe that the sixth ICS of thoracoscopic port may sometimes offer better view of the bronchovascular stoma.
There was no mature procedure or surgical process for VATS double sleeve lobectomy before. We summarized our practice of this operation as the “hollow-out” process. After exploring the chest, the hilum and mediastinal lymph nodes were dissected first, including level 4, 5, 6, 7, 9 in the left side, and level 2, 3, 4, 7, 9 in the right side. Connective tissues surrounding the hilum structures were fully removed to expose the vessels and the bronchus. The “hollow-out” process enabled the blockade of the pulmonary artery. In addition, removal of the lymph nodes at first also avoided retracting the anastomotic stoma if we performed the lymph node dissection after bronchovascular reconstruction.
Another important issue that assures the safety of the operation is the appropriate instruments for vascular clamping. The long handle of traditional vascular clamps used in a utility incision may cause inconvenience during suturing. In Huang’s multi-center case series, they made the fourth port to clamp the pulmonary artery (13). Atraumatic Bulldog Clamps placed by an endoscopic applicator were firstly chosen for VATS pulmonary artery clamping in our center. This kind of clamps avoided the interference caused by the handle of standard vascular clamps. The curved Bulldog Clamps were more convenient for use when comparing with the straight clamps. Considerable concern was expressed with regard to the safety of the clamps used for vascular clamping. Displacement of the clamp would be disastrous to the procedure. The Bulldog clamp we chose to clamp the pulmonary artery had been used to clamp the renal artery and vein during laparoscopic partial nephrectomy (15). It has been confirmed reliable for endoscopic use. Otherwise, a D’Amico DeBakey Clamp was prepared during the operation to prevent and handle massive bleeding caused by the displacement of the Bulldogs.
Performing the anastomosis is the main technical difficulty during VATS sleeve lobectomy. Through thoracoscopic view, the main bronchus is anatomically beneath the pulmonary artery on both sides. Therefore, the artery must be retracted when VATS bronchial reconstruction was performed. When performing bronchovascular double sleeve resection, both the artery and bronchus were cut down, enabling a better view for bronchial reconstruction. During open bronchovascular sleeve lobectomy, pulmonary artery reconstruction is usually recommended to be performed after completion of the bronchial anastomosis in order to avoid retracting the freshly sewn fragile artery (16-18). With the thoracoscopic approach, we also prefer sewing up the bronchus first. This may prolong the clamping time of pulmonary artery. Therefore, heparin was administered before arterial clamping to avoid thrombosis in the circulatory-arrested lobe.
As for anastomotic techniques, the bronchial anastomosis can be performed using both interrupted suture and running suture while the pulmonary artery is usually reconstructed using running 5-0 monofilament nonabsorbable sutures (18). Postoperative complications after bronchial anastomosis with running or interrupted suture are similar (19). We prefer to perform running suture using monofilament nonabsorbable sutures during open bronchial sleeve lobectomy. The use of running suture during VATS bronchial anastomosis was firstly introduced by our team previously (6). When performing VATS bronchovascular anastomosis, we also prefer the running suture rather than the interrupted suture for it’s more convenient and time-saving. During suturing, we applied a pair of endoscopic Kelly forceps and a long handle needle holder through the assistant and utility port at first. After a few cases of practice, we changed to use an endoscopic needle holder and a long handle needle holder during suturing. We named this as the “two-needle-holder suturing technique”. This technique enabled us to perform the anastomosis through different directions without exchanging the needle holder among different ports.
Bronchial pleural fistula and broncho arterial fistula are also important issues that should be taken into account when performing a VATS bronchovascular double sleeve lobectomy. Broncho arterial fistula is rare but usually a lethal complication after double sleeve lobectomy. It is necessary to separate the bronchus and pulmonary artery after the surgery, in order to minimize the influence between the two anastomosis. Different kinds of autogenous tissue flaps, such as intercostal muscle flap, pleural or pericardial flap, can be applied to cover the bronchial anastomosis and prevent the fistula in open surgery (20,21). Some absorbable sealing materials, such as polyglycolic acid felt, have been applied to prevent the complications caused by bronchial anastomosis insufficiency after surgery (22). After VATS bronchovascular reconstruction, we also took the measure to prevent the problems due to bronchial anastomosis insufficiency. The bronchial anastomosis was covered with a piece of polyglycolic acid felt which provided a mechanical cushion between the two newly reconstructed structures. Unfortunately, one of the patients died from hemoptysis 50 days after surgery, which may be caused by broncho arterial fistula.
With regard to the time of the operation, the first experience of VATS bronchovascular double sleeve lobectomy was completed in 8 hours. In the next six operations, surgical duration decreased to approximately four to 6 hours. Prolonged operative time is an important predictive factor of postoperative pulmonary complications (23,24). Compared with open lobectomy, thoracoscope takes a significantly longer surgery time or is considered insignificant in most studies, as summarized by Yan et al. (25). The operative time of the first experience of VATS bronchovascular double sleeve lobectomy was much longer compared with our routine open bronchovascular sleeve lobectomy. The new surgical procedure presents a learning curve. For the first few cases, it took us longer time to explore the surgical process, and suitable instruments because there was no mature experience we could refer to. We believe that the surgical duration of VATS bronchovascular double sleeve lobectomy will be shortened with the improvement of surgical procedure, more skilled surgical techniques and increased operations.
All patients were discharged when recovery went well. Two patients had pneumonia after surgery, which prolonged their postoperative hospital stay. The long-term outcome of patients who underwent VATS bronchovascular double sleeve lobectomy is also an important concern of this newly emerging operation. Among these seven patients, one died of hemoptysis 50 days after surgery, and one patient died from metastatic lung cancer within 2 years. The other patients are alive with longest follow-up of 58 months. In Huang’s multi-center case series, the longest follow-up was 26 months without any local recurrences or distant metastasis (13). However, due to the small-sized sample and relatively short-term follow-up, this procedure needs further assessment.
Conclusions
VATS bronchovascular double sleeve lobectomy is technically difficult, but feasible. However, the surgical incisions, procedures, and specific instruments have been improved obviously. Operative time is expected to be reduced with skilled techniques. This operation can be accomplished by skilled thoracoscopic surgeons in experienced center. More data are encouraged to assess the long-term outcomes of this new procedure.
Acknowledgments
Funding: This study was supported by the Key Science and Technology Program of Sichuan Province, People’s Republic of China (2013SZ0005 and 2014SZ0148, both to Dr. L Liu).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2017.12.01). Lunxu Liu serves as an unpaid Editor-in-Chief of Video-Assisted Thoracic Surgery. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Use of the data for this study was approved by the institutional review board (IRB) of West China Hospital (NO. 2016-98). Because of this is a retrospective archive study, informed consent from the patients was waived by the IRB.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Santambrogio L, Cioffi U, De Simone M, et al. Video-Assisted Sleeve Lobectomy for Mucoepidermoid Carcinoma of the Left Lower Lobar Bronchus: A Case Report. Chest 2002;121:635-6. [Crossref] [PubMed]
- Mahtabifard A, Fuller CB, McKenna RJ Jr. Video-assisted thoracic surgery sleeve lobectomy: a case series. Ann Thorac Surg 2008;85:S729-32. [Crossref] [PubMed]
- DeArmond DT, Mahtabifard A, Fuller CB, et al. Photodynamic therapy followed by thoracoscopic sleeve lobectomy for locally advanced lung cancer. Ann Thorac Surg 2008;85:e24-6. [Crossref] [PubMed]
- Agasthian T. Initial experience with video-assisted thoracoscopic bronchoplasty. Eur J Cardiothorac Surg 2013;44:616-23. [Crossref] [PubMed]
- Li Y, Wang J. Video-assisted thoracoscopic surgery sleeve lobectomy with bronchoplasty: an improved operative technique. Eur J Cardiothorac Surg 2013;44:1108-12. [Crossref] [PubMed]
- Mei J, Pu Q, Liao H, et al. Initial Experience of Video-Assisted Thoracic Surgery Left Upper Sleeve Lobectomy for Lung Cancer: Case Report and Literature Review. Thorac Cancer 2012;3:348-52. [Crossref] [PubMed]
- Mei JD, Pu Q, Ma L, et al. Improving the Procedures of Video-assisted Thoracoscopic Surgery Bronchial Sleeve Lobectomy for Lung Cancer. Sichuan Da Xue Xue Bao Yi Xue Ban 2013;44:114-8. [PubMed]
- Liu L, Mei J, Pu Q, et al. Thoracoscopic bronchovascular double sleeve lobectomy for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;46:493-5. [Crossref] [PubMed]
- Nakanishi R, Yamashita T, Oka S. Initial experience of video-assisted thoracic surgery lobectomy with partial removal of the pulmonary artery. Interact Cardiovasc Thorac Surg 2008;7:996-1000. [Crossref] [PubMed]
- Zhang Z, Huang J, Yin R, et al. A new technique for partial removal of the pulmonary artery in video-assisted thoracic surgical lobectomy. J Thorac Cardiovasc Surg 2012;144:512-4. [Crossref] [PubMed]
- Mei J, Pu Q, Liao H, et al. A novel method for troubleshooting vascular injury during anatomic thoracoscopic pulmonary resection without conversion to thoracotomy. Surg Endosc 2013;27:530-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Double sleeve uniportal video-assisted thoracoscopic lobectomy for non-small cell lung cancer. Ann Cardiothorac Surg 2014;3:E2 [PubMed]
- Huang J, Li J, Qiu Y, et al. Thoracoscopic double sleeve lobectomy in 13 patients: a series report from multi-centers. J Thorac Dis 2015;7:834-42. [PubMed]
- Gonzalez-Rivas D, Yang Y, Stupnik T, et al. Uniportal video-assisted thoracoscopic bronchovascular, tracheal and carinal sleeve resections. Eur J Cardiothorac Surg 2016;49:i6-16. [PubMed]
- Andonian S, Janetschek G, Lee BR. Laparoscopic partial nephrectomy: an update on contemporary issues. Urol Clin North Am 2008;35:385-96. vii. [Crossref] [PubMed]
- Rendina EA, Venuta F, De Giacomo T, et al. Sleeve resection and prosthetic reconstruction of the pulmonary artery for lung cancer. Ann Thorac Surg 1999;68:995-1001; discussion 1001-2. [Crossref] [PubMed]
- Venuta F, Ciccone AM, Anile M, et al. Reconstruction of the pulmonary artery for lung cancer: long-term results. J Thorac Cardiovasc Surg 2009;138:1185-91. [Crossref] [PubMed]
- Ibrahim M, Maurizi G, Venuta F, et al. Reconstruction of the bronchus and pulmonary artery. Thorac Surg Clin 2013;23:337-47. [Crossref] [PubMed]
- Kutlu CA, Goldstraw P. Tracheobronchial sleeve resection with the use of a continuous anastomosis: results of one hundred consecutive cases. J Thorac Cardiovasc Surg 1999;117:1112-7. [Crossref] [PubMed]
- Rendina EA, Venuta F, Ricci P, et al. Protection and revascularization of bronchial anastomoses by the intercostal pedicle flap. J Thorac Cardiovasc Surg 1994;107:1251-4. [PubMed]
- Yildizeli B, Fadel E, Mussot S, et al. Morbidity, mortality, and long-term survival after sleeve lobectomy for non-small cell lung cancer. Eur J Cardiothorac Surg 2007;31:95-102. [Crossref] [PubMed]
- Kamiyoshihara M, Nagashima T, Igai H, et al. Video-assisted thoracic lobectomy with bronchoplasty for lung cancer, with special reference to methodology. Interact Cardiovasc Thorac Surg 2011;12:534-8. [Crossref] [PubMed]
- Stéphan F, Boucheseiche S, Hollande J, et al. Pulmonary complications following lung resection: a comprehensive analysis of incidence and possible risk factors. Chest 2000;118:1263-70. [Crossref] [PubMed]
- Nan DN, Fernández-Ayala M, Fariñas-Alvarez C, et al. Nosocomial infection after lung surgery: incidence and risk factors. Chest 2005;128:2647-52. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
Cite this article as: Mei J, Guo C, Pu Q, Ma L, Liu C, Zhu Y, Liao H, Liu L. Video-assisted thoracic surgery double sleeve lobectomy for non-small cell lung cancer: a report of seven cases. Video-assist Thorac Surg 2018;3:1.


