A 2.5 cm anterior incision and a 12 mm subxiphoid port to perform a left lower VATS lobectomy: an attempt to improve uniportal VATS technique
Introduction
Video-Assisted Thoracoscopic Surgery (VATS) lobectomy is actually considered the gold standard approach in the treatment of non-small cell lung cancer for his improved perioperative morbidity and superior perioperative survival compared to thoracotomy. Different techniques have been described: UVATS was first described in 2004 by Rocco and colleagues (1); Gonzales-Rivas used posteriorly this strategy to perform a lobectomy (2-6); a two-incision VATS for anatomic pulmonary resection was performed by different groups (7-12), and a standardised three-port approach was described by different authors (13-17). Many authors identify UVATS lobectomy with the least possible access trauma of any VATS lobectomy technique: we are convinced that with the use of the thoracic outlet this aspect can be further improved (18,19).
Patient selection and workup
As UVATS lobectomy, the technique we present is not only indicated for initial stages of NSCLC.
Preoperative evaluation of patient includes:
- Pulmonary function testing;
- Computerized tomography (CT);
- Positron emission tomography (PET/CT);
- Flexible bronchoscopy;
- EBUS (central tumour and/or hilar/mediastinal lymp nodes hypermetabolic on PET);
- mediastinoscopy (central tumour, tumour >3 cm, mainly adenocarcinoma with high fludeoxyglucose uptake, mediastinal lymp nodes hypermetabolic on PET and negative on EBUS).
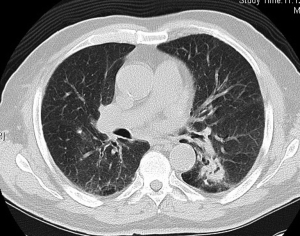
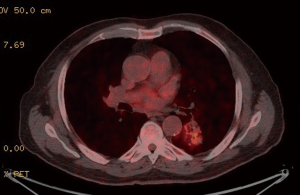
Preoperative CT demonstrated a left lower lobe with a solitary hilar spiculated nodule −35 mm diameter (Figure 1). PET-CT showed a hypermetabolic nodule in the left lower lobe with mean standardized uptake value (SUV) equal to 4.8 (Figure 2). Mediastinal involvement was discarded. Relevant data of respiratory function testing:
- FEV1 equal to 2.5 litres (70% predicted);
- DLCO equal to 45% predicted.
Pre-operative preparation
A lateral decubitus is the standard position in order to perform a lobectomy with our technique. The surgeon is placed in front of the patient, and the assistant on the opposite side. It is important to stress that we preserve the main advantage of UVATS technique, the possibility to maintain step by step the same angle of vision of an open lobectomy.
Equipment preference card
Useful kit:
- COVIDIEN SILS™ PORT 12 mm;
- COVIDIEN SILS™ DISSECTOR 36 cm length;
- COVIDIEN SILS™ CLINCH 36 cm length;
- COVIDIEN SILS™ HOOK 36 cm length;
- LigaSure
TM Maryland Jaw 23 cm (COVIDIEN, Mansfield, MA, USA); - COVIDIEN Endo-Gia Stapler with curved-tip stapler technology and tri-staple cartridges;
- COVIDIEN Versa-One
TM Blunt Trocar 12 mm; - Carbon Dioxide insufflation (8–10 mmHg of pressure);
- 5 mm sucker;
- 5 mm endopeanuts;
- COVIDIEN 12 mm Endobag
TM Specimen Retrieval System; - Olympus Ultra Telescope 10 mm (45 degree).
Various types of wound protectors are described to perform a UVATS lobectomy. We have replaced these devices by the COVIDIEN SILS™ PORT in order to improve vision using carbon dioxide insufflation.
Procedure
Fixed steps in the our surgical procedure are listed below:
- General anaesthesia with double lumen intubation
- Lateral decubitus
- Anterior incision −2.5 cm in length—at the level of the 5th intercostal space.
Left lower lobectomy
In laparoscopic surgery the use of the COVIDIEN SILS™ PORT is well established; in thoracoscopic surgery its use is not common and it is basically applied for the treatment of primary spontaneous pneumothorax (18,19). We described for the first time the use of the COVIDIEN SILS™ PORT in a lobectomy procedure.
We placed the COVIDIEN SILS™ PORT in the 2.5 cm anterior incision so that the multiple ports had the following orientation:
- Twelve mm access channel at the top to introduce the thoracoscope into the pleural cavity;
- 5 mm access channels at the bottom to introduce into the pleural cavity the described instruments;
Operative steps for left lower lobectomy:
- Pulmonary ligament;
- Inferior vein;
- Inferior bronchus;
- Artery (superior and basilar segment) and fissure: from anterior to posterior.
The lobectomy is not technically different depending on whether the fissure is complete or not. The subxiphoid port is at the end of the oblique fissure and it is very convenient for securely stapling the artery and the fissure together with the tri-staple purple cartridges.
Before performing a systematic lymph node dissection, the lobe is removed into a protective bag.
Removal of the specimen from an intercostal space could increase postoperative pain. Ribs are immobile and held together by intercostal muscles and therefore a large distance of intercostal muscle must be divided to allow the ribs to separate.
In the subxiphoid region the line alba can be incised and the rectus abdominis muscles separate very easily and painlessly.
For that reason we take the specimen out from the subxiphoid port: this technical detail alone makes a difference, especially for larger tumours.
Final step consists in infiltrating the intercostal spaces with bupivacaine under thoracoscopic view. This manoeuvre is usually performed from the second intercostal space to the utility port level to minimize the pain caused by the chest tube.
A single 28F chest tube is placed through the subxiphoid port: pain comes from the intercostal nerves so this technical detail could be also important for decreasing postoperative pain.
A paravertebral catheter is routinely inserted for a minimum of 24 hours: continuous infusion, via an elastomeric pump, of 1.25 mg/mL levobupivacaine plus 1 µg/mL fentanil, set at a rate of 5 mL/h.
Lymphadenectomy
With the camera in the upper part of the utility port, a systematic lymph node dissection was carried on.
Systematic steps:
- Paratracheal dissection with the patient in the anti-Trendelenburg position, thereby making the lung drop or “fall down”
- Subcarinal lymph nodes dissection with the patient in the Trendelenburg position, so that an optimal exposure is achieved
- Hilar and N1 lymph nodes dissection: it could be effectively performed moving the table in a posterior rotation
Role of team members
A team approach is essential to achieve excellence and to maintain efficiency in the operating theatre.
The surgical team consists of:
- Surgeon and assistant
- Anaesthesiologist
- Scrub nurse
- Circulating nurse
Surgeon’s skills:
- Leadership
- Ensuring coordination of care in the theatre
- Resolving unexpected intraoperative events and complications
The surgeon should always review emergency procedures, and blood availability and instruments for an unplanned conversion to thoracotomy.
Anaesthesiologist’s responsibilities:
- Monitoring and maintaining patient hemodynamic and pulmonary stability
- Performing single lung ventilation to avoid intraoperative hypoxemia
- Ensuring a close communication regarding fluid and blood pressure management, essential in case of unexpected complications (bleeding and/or emergency pneumonectomy)
Postoperative management
The conditioning factors are:
- Pain management;
- Aggressive pulmonary physiotherapy;
- Early mobilization.
The anaesthetist retired the mechanical ventilation in the theatre and the patient received a close monitoring in the intensive care unit during the first 24 hours.
The pleural drain was retired after 24 hours.
Before removing the chest tube, pain management was via the paravertebral catheter and then with oral analgesics.
Tips, tricks and pitfalls
- Location of incision: placement of the incision should be variable: depending on performing an upper or a lower lobectomy, it could be located between the fourth and the sixth intercostal spaces. It is my contention that the presence of the subxiphoid port limits this variability and the fifth intercostal space turns out to be an optimal approach in all the cases;
- The subxiphoid port is very convenient for securely stapling all the hilar structures and it is very unusual to have to resort to using vascular clips due to no angle for staplers;
- In case of incomplete fissure with very thick tissue, a black cartridge (Endo GIA™ 60 mm Articulating Extra-Thick Reload with Tri-Staple™ Technology) can be used to provide superior staple formation and strength, and to reduce postoperative air leak;
- Using an optic with chip on tip and/or an articulating HD videoscope (Olympus Articulating-Tip ENDOEYE FLEX LTF-VH) could be improve the vision and avoid compromising dexterity.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2017.02.07). VP serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from Oct 2016 to Mar 2019. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [Crossref] [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Liu CY, Lin CS, Shih CH, et al. Single-port video-assisted thoracoscopic surgery for lung cancer. J Thorac Dis 2014;6:14-21. [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Evolving from conventional video-assisted thoracoscopic lobectomy to uniportal: the story behind the evolution. J Thorac Dis 2014;6:S599-603. [PubMed]
- Zhu Y, Xu GB, Lei CG, et al. Thoracic surgery: single-port video-assisted thoracoscopic lobectomy. Ann Transl Med 2015;3:143. [PubMed]
- Burfeind WR, D'Amico TA. Thoracoscopic lobectomy. Oper Tech Thorac Cardiovasc Surg 2004;9:98-114. [Crossref]
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- D'Amico TA, Niland J, Mamet R, et al. Efficacy of mediastinal lymph node dissection during lobectomy for lung cancer by thoracoscopy and thoracotomy. Ann Thorac Surg 2011;92:226-31; discussion 231-2. [Crossref] [PubMed]
- Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487-93. [Crossref] [PubMed]
- Kara HV, Balderson SS, D'Amico TA. Modified uniportal video-assisted thoracoscopic lobectomy: Duke approach. Ann Thorac Surg 2014;98:2239-41. [Crossref] [PubMed]
- McKenna RJ Jr. Lobectomy by video-assisted thoracic surgery with mediastinal node sampling for lung cancer. J Thorac Cardiovasc Surg 1994;107:879-81; discussion 881-2. [PubMed]
- McKenna RJ Jr, Wolf RK, Brenner M, et al. Is lobectomy by video-assisted thoracic surgery an adequate cancer operation? Ann Thorac Surg 1998;66:1903-8. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Walker WS, Codispoti M, Soon SY, et al. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg 2003;23:397-402. [Crossref] [PubMed]
- Hansen HJ, Petersen RH. Video-assisted thoracoscopic lobectomy using a standardized three-port anterior approach - The Copenhagen experience. Ann Cardiothorac Surg 2012;1:70-6. [PubMed]
- Perna V, Carvajal AF, Torrecilla JA, et al. Uniportal video-assisted thoracoscopic lobectomy versus other video-assisted thoracoscopic lobectomy techniques: a randomized study. Eur J Cardiothorac Surg 2016;50:411-5. [Crossref] [PubMed]
- Ismail NA, Elsaegh M, Dunning J. Novel Techniques in Video-assisted Thoracic Surgery (VATS) Lobectomy. Surg Technol Int 2015;26:206-9. [PubMed]
Cite this article as: Perna V, Carvajal A, Torrecilla JA, Gigirey O, Mora LC, Cano P, Perello S, Diaz R. A 2.5 cm anterior incision and a 12 mm subxiphoid port to perform a left lower VATS lobectomy: an attempt to improve uniportal VATS technique. Video-assist Thorac Surg 2017;2:20.