Video-assisted thoracic surgery for esophagectomy: evolution and prosperity
Introduction
Esophageal cancer has very poor prognosis and decrease patients’ quality of life (QoL) dramatically (1). According to the GLOBOCAN project of World Health Organization (WHO), esophageal cancer is now the eighth most common cancer worldwide and the sixth common cause of death from cancer (2). Eighty percent of worldwide esophageal cancer occur in less developed regions such as eastern Asia and Africa (2). In 2015, the estimated new cancer cases and deaths in China was 477,900 and 375,000 respectively, most of them was squamous cell carcinoma (SCC) (1). Although SCC is the most common esophageal malignancy worldwide, the incidence of esophageal adenocarcinoma also has been increasing rapidly in Western countries (3). According to previous study, global incidence of esophageal cancer has increased by 50% in the past two decades (4).
For the patients with esophageal cancer without distant metastasis, surgical resection is the most effective treatment, despite great improvement in chemotherapy and radiotherapy in the past decades. Since Franz Torek successfully conducted the first transthoracic esophagectomy in 1913 (5), various surgical approaches have been established to resect the esophagus and reconstruct the alimentary canal, such as transhiatal esophagectomy (THE), McKeown esophagectomy, Ivor-Lewis esophagectomy and Sweet esophagectomy (6-9).
However, esophagectomy is one of the most invasive surgeries. It involves two or three compartment dissection, radical lymphadenectomy, and upper gastrointestinal tract reconstruction. As a result, the traditional open esophagectomy is associated with considerable morbidity and mortality, with complication rates ranging from 26% to 41% and perioperative mortality rates ranging from 4% to 10% (10).
In the past two decades, the technical advancements and development of endoscopic equipment in thoracoscopic surgery have resulted in the popularity of video-assisted thoracic surgery (VATS). In 1992, Dr. Cuschieri first reported thoracoscopic esophagectomy as minimally invasive esophagectomy (MIE) (11). Since then, more and more surgeons have adopted this operation in patients with esophageal cancer (11-13). Recently the development of linear stapling device, ultrasonic scalpel, anastomat and vessel-sealing systems further contributed to the evolution of MIE (14,15).
In this study, we review previous literature on minimally invasive esophagectomy, aiming to highlight the evolution and prosperity of MIE and assess the short- and long-term outcomes of MIE compared with traditional open esophagectomy.
Historical note
Franz Torek was the first surgeon who successfully implemented transthoracic esophagectomy in 1913 (5). And the first esophagectomy with esophagogastric reconstruction was reported by Ohsawa in Japan in 1933 (16). After that Adams and Phemister adopt this surgery in the United States in 1939 (17). In 1946, Ivor Lewis, surgeon of North Middlesex County Hospital, also known as the founder of Ivor-Lewis esophagectomy, conducted transthoracic resection of the esophagus via laparotomy and right thoracotomy routinely (8). In 1974, McKeown implemented the 3-incision esophagectomy which bears his name (18). The tri-incisional esophagectomy is similar in concept to an Ivor-Lewis procedure, but it tends to be used for esophageal lesions that in the proximal esophagus. Moreover, rather than thoracic esophagogastric anastomosis, the anastomosis of this procedure is completed in left neck. Other variations of the transthoracic esophagectomy include Sweet procedure popularized by Richard Sweet in 1945 (9) and the wide en-bloc esophagectomy popularized by Skinner in 1983 (19). The milestones of esophagectomy evolution was shown in Table 1.
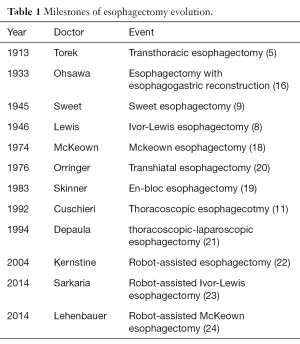
Full table
Evolution of minimally invasive esophagectomy
“We have come to realize that the Torek type of operation will possibly prolong the patient’s life, and certainly prolong his misery.”——Ivor Lewis, 1946, Royal College of Surgeons, London.
In 1946, Ivor Lewis gave a lecture at the Royal College of Surgeons in London, talking about surgical treatment for esophageal cancer (8). As we know, Torek was the first surgeon who successfully conducted transthoracic esophagectomy (5). The excerpt above was quite an important part of this lecture, emphasizing the huge trauma caused by esophagectomy. As a radical surgery for esophageal cancer, esophagectomy certainly can improve patients’ survival rate, but it did cause much misery to them simultaneously. It could be deduced from this lecture that surgeons at that time faced with a severe problem caused by esophagectomy trauma, and Dr. Ivor Lewis valued minimally invasive esophagectomy a lot.
Transhiatal esophagectomy
Traditional esophagectomy involved abdominal cavity dissection, thoracic cavity dissection, with or without cervical incision. Aiming to reduce the surgical trauma caused by traditional esophagectomy, Mark Orringer conducted the first transhiatal esophagectomy in 1976 (20). This surgical approach is quite different from previously mentioned approaches, removing the esophagus through the diaphragmatic hiatus without thoracotomy. The brief procedures are as follows. First, an upper abdominal incision from xiphisternum to the navel is made. The surgeon then mobilizes the esophagus by working upward through the diaphragmatic hiatus. With an addition incision on the left neck, the surgeon completes the mobilization of the esophagus, removes it, and moves the stomach upward through the hiatus and into the chest until its extremity appears in the neck incision. The last step is the cervical esophagogastric anastomosis (25). Without thoracotomy, THE approach could be regarded as the first endeavor to reduce surgical invasion.
In a study based on Surveillance, Epidemiology, and End Results (SEER)—Medicare linked database (1992 to 2002), 225 patients underwent transhiatal esophagectomy and 643 patients underwent transthoracic esophagectomy (26). As a result, lower operative mortality rate was observed in THE group compared with transthoracic group (6.7% vs. 13.1%, P=0.009). And the adjusted 5-year overall survival rate was not compromised. However, THE group was more likely to require endoscopic dilatation within 6 months of surgery (43.1% vs. 34.5% for transthoracic group, P<0.02). In another meta-analysis study, totally 24 studies compared transthoracic with transhiatal esophagectomy were involved (27). This study showed less blood loss in THE group compared with transthoracic group (728±438 vs. 1001±575 mL, P<0.0001), less operation time in randomized studies (3.5 vs. 5.2 hours, P<0.0001), less ICU stay (9.1±5.3 vs. 11.2±6.2 days, P<0.0001) and less hospital stay (17.8±10.3 vs. 21.0±16.2 days, P<0.0001). Another study published in The New England Journal of Medicine, investigated the comparison between THE and extended transthoracic resection for esophageal adenocarcinoma patients (28). This multi-center, randomized controlled trial showed THE group not only possessed shorter surgery time, less ICU stay and hospital stay, but also less pulmonary complications (27% vs. 57%, P<0.0001), less chylous leakage (2% vs. 10%, P=0.020). However, for transhiatal and transthoracic esophagectomy group, estimated 5-year disease-free survival rates were 27 percent and 39 percent, respectively, whereas 5-year overall survival rates were 29 percent and 39 percent (without statistic significance). This was probably because the number of lymph node yields was significant lower in THE group (16±9 vs. 31±14, P<0.0001). For esophageal cancer patients, extended resection is believed to reduce the rate of local–regional recurrence, thereby increasing the quality of life and prolonging disease-free and overall survival.
According to these studies, there is no controversy that THE approach can decrease surgical trauma as well as early postoperative risk to esophageal cancer patients. But without formal lymphadenectomy, THE approach yields significant less lymph nodes compared with traditional transthoracic esophagectomy, and consequently tend to unsatisfactory long-term survival. This is the main controversy, and probably the main disadvantage of transhiatal esophagectomy.
Emergence and prosperity, maturity are subtitles of video-assisted minimally invasive esophagectomy
Emergence and prosperity
Transhiatal esophagectomy remains disadvantages in lymph nodes resection and may induce long-term poor prognosis consequently (28). And the requirement for minimally invasive esophagectomy still remains. With the development of endoscopy technique, the first MIE using a right thoracoscopic approach was initially conducted by Cushieri in 1992 (29,30), and the first combined thoracoscopic-laparoscopic esophagectomy was conducted 2 years later by DePaula (21). Since then thoracoscopic esophagectomy had attracted increasing attention as an alternative approach to open surgery to reduce surgical invasiveness (31-33), and the minimally invasive esophagectomy had entered a new era.
Thoracoscopic esophagectomy poses an important challenge for thoracic surgeons as it is a sophisticated technique to be mastered. For all surgeons, competence at open surgery does not invariably translate into endoscopic skills. It does require an appreciable learning curve, especially the thoracic esophagogastric anastomosis procedure and bilateral recurrent laryngeal nerve (RLN) lymphadenectomy (34). In this study, totally 109 patients were chronologically subcategorized into early group (26 patients), middle group (62 patients) and 21 most recent cases as the late group. As a result, both the middle and the late groups had significantly improved results compared with the early group in intrathoracic anastomosis time (P<0.001, P<0.001), abdominal operating time (P<0.001, P<0.001), total operating time (P<0.001, P<0.012), and blood loss quantity (P<0.001, P<0.015). Compared with the early group, both the middle and the late group had significantly increased lymph node retrieval; this was sorted into the thoracic (P<0.001, P<0.001), abdominal (P<0.019, P<0.004), and bilateral RLN lymph nodes (P<0.001, P<0.001). This study indicates that learning curve for minimally invasive esophagectomy does exist, but outcome measurements of MIE are improving with the mastery of thoracoscopic esophagectomy technique, with no apparent compromise of oncological outcomes.
To further evaluate the reliability and efficiency of thoracoscopic esophagectomy, Taguchi and his colleagues conducted a retrospective study comparing the differences of surgery parameter, postoperative pulmonary function, quality of life and overall survival between thoracoscopic and open three-field esophagectomy (35). From July 1996 to August 1998, totally 51 patients with esophageal cancer underwent thoracic esophagectomy with radical lymphadenectomy by posterolateral thoracotomy (29 cases) or thoracoscopic surgery (22 cases). As a result, pre-to-postoperative change in vital capacity was 74.3%±10.6% in the thoracotomy group and 84.9%±10.4% in the thoracoscopy group (P=0.021). Maximum oxygen uptake was similar, but dyspnea was the more common factor limiting exercise tolerance postoperatively in the thoracotomy group. Change in pre-to-postoperative performance status was 1.20±0.62 in the thoracotomy group and 0.55±0.51 in the thoracoscopy group (P<0.001). Five-year survival rate in the two groups was similar (P=0.37). The study indicated that VATS helped reduce postoperative restrictive pulmonary dysfunction, possibly by minimizing chest wall trauma, and better preserved performance status compared with traditional open surgery, without compromising long-term survival in esophageal cancer patients. The author also published another study reported that fewer pulmonary complications occur after VATS than after open surgery once the plateau of learning the technique has been achieved (36).
Maturity
With the development and popularization of VATS technique, more and more centers adopted thoracoscopic esophagectomy (15,37-40). Nowadays, two-phase Ivor-Lewis esophagectomy and three-phase McKeown esophagectomy are the main surgical approach of minimally invasive esophagectomy. For hybrid MIE (hMIE), only laparoscopy or thoracoscope was used during the whole procedure. For totally MIE (tMIE), laparoscopic-thoracoscopic procedures were performed during abdominal and thoracic stage. The surgical procedures may differ in different centers. Briefly, the patient is initially supine for the laparoscopic stage, which includes gastric mobilization with abdominal lymphadenectomy, gastric resection, and intra-abdominal gastric conduit formation. This is followed by thoracoscopic esophageal mobilization and mediastinal lymphadenectomy, which includes the subcarinal nodes and all of the paraesophageal nodes. Then the thoracic or cervical esophagogastric anastomosis is created to finish the digestive tract reconstruction.
The largest sample size study of MIE was reported by Luketich (15) in 2012. In this study, from 1996 to 2011, totally 1,011 consecutive patients received MIE with cervical anastomosis (MIE-McKeown, 481) or intrathoracic anastomosis (MIE-Ivor Lewis, 530) were involved. Initially, they conducted the laparoscopic/thoracoscopic 3-incision McKeown procedure with cervical esophagogastric anastomosis. Then they converted to a laparoscopic/thoracoscopic Ivor-Lewis procedure with intrathoracic esophagogastric anastomosis because of less morbidity occurred in this group. In this series, the combined 30-day and in-hospital mortality for the entire group was 2.8%. Although the 30-day mortality did not possess significant difference between the MIE-Ivor Lewis and the MIE-McKeown groups (0.9% vs. 2.5%, P=0.83), there was a significant difference in the combined 30-day and in-hospital mortality between the groups (1.7% vs. 3.95%, P=0.035). The overall incidence of postoperative adverse events did not differ between the MIE-Ivor-Lewis and the MIE-McKeown group. However, laryngeal nerve paralysis was more common in patients undergoing the MIE-McKeown procedure (8% vs. 1%, P<0.001) as was the development of acute respiratory distress syndrome (4% vs. 2%, P=0.030). This series demonstrated that the MIE can be performed with acceptable mortality and low morbidity for esophageal cancer patients. Moreover, it suggests that the MIE-Ivor Lewis approach appears to be better than the MIE-McKeown technique. Because the investigators did not routinely obtain contrast esophagrams in all patients, an anastomotic leak rate was not reported. However, 5% of the patients in the series developed an anastomotic leak requiring operative intervention, and this did not differ between the 2 different MIE techniques.
Another profound study was a multicenter, open-label, randomized control trial published in Lancet in 2012 (41). In this study, Biere and his colleagues randomly assigned 56 patients to the open esophagectomy group and 59 to the minimally invasive esophagectomy group. As a result, 16 (29%) patients in the open esophagectomy group had pulmonary infection in the first 2 weeks compared with 5 (9%) in the minimally invasive group (P=0.005). Nineteen (34%) patients in the open esophagectomy group had in-hospital pulmonary infection compared with 7 (12%) in the minimally invasive group (P=0.005). And the hospital stay was shorter in MIE group (11 vs. 14 days, P=0.044). Because of less surgical trauma in MIE group, visual analogue scale pain score was dramatically decreased win MIE group (P=0.001). Additionally, there was a significant difference of vocal-cord paralysis between MIE group and open group (2% vs. 14%, P=0.012). For in-hospital mortality, these two groups didn’t have a significant difference. These findings provide evidence for the short-term benefits of minimally invasive esophagectomy for patients with resectable esophageal cancer, such as less pulmonary infection, less surgical pain and less vocal-cord paralysis.
According to the data from a population-based UK national study, there has been a steady increase in the uptake of MIE from 6.2% in 2005 to 24.7% in 2009 (42). With the popularization of MIE, more and more meta-analysis studies were published to compare the differences between MIE and open esophagectomy (43-47). It is generally believed that the advantages of MIE include shorter hospital stay, less postoperative pain and less pulmonary complications, without compromising long-term survival rate.
With the maturity of video-assisted minimally invasive esophagectomy, some innovative approaches emerge. In 2015, Dr. Hecheng Li first reported totally minimally invasive Ivor-Lewis esophagectomy (MIIE) with single-utility incision VATS in mid-lower esophageal cancer patients (48). This new technique was successfully conducted in 12 patients, without in-hospital mortality. For these patients, the average operative duration was 275.4±31.2 minutes, and the mean blood loss was 220±94.9 mL. One patient developed late-stage anastomotic leakage. The average thoracic or abdominal lymph node yield was 14.7±8.8 and 6.3±5.7, respectively. No statistically significant differences were identified between single-utility MIIE and traditional four-port MIIE. In another randomized control trial conducted by Dr. Yin Li in 2014, 148 continuous esophageal cancer patients were recruited (49). They randomized assigned 72 patients to “non-tube no fasting” group and 76 patients to late oral feeding group. All patients underwent laparoscopic/thoracoscopic esophagectomy. Patients in the “non-tube no fasting” group started oral nutrition on postoperative day (POD) 1 at will without a nutrition tube and fasting. In interim analyses, the anastomotic leakage rate was 2.8% for the “non-tube no feasting” group, which is significantly lower than that observed for mechanically stapled anastomosis and fasting for 7 days during the same period in other medical groups (n=92), (2.8% vs. 10.9%, P=0.048). The post-operation hospital stay (7.6±2.2 vs. 12.1±3.7, P<0.01) is dramatically decreased. The Health-related quality of life (HRQL) mean scores obtained 3 months post operation were significantly better than the late oral feeding, including those for reflux (14.07±14.86 vs. 22.96±17.73, P=0.048) and dysphagia (15.56±15.33 vs. 23.70±16.95, P=0.047). Additionally, the stricture rate is lower in the “non-tube no feasting” group.
VATS occupy an important position in minimally invasive esophagectomy. These published studies indicated that after decades of development, minimally invasive esophagectomy has come to an age of maturity.
Robotic-assisted esophagectomy
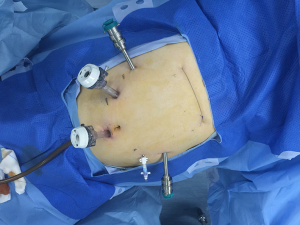
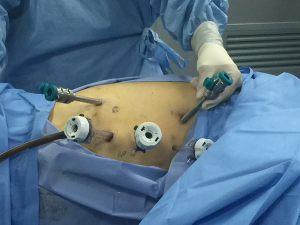
Although the first surgical robot system was constructed in 1983, it was not until 1992 that the first robotic operation, a prostatectomy, was performed. Since then, robotic assisted surgery has developed rapidly. With advantages including clearer and intuitive enhanced three-dimensional visualization, improved magnification, and a greater range of instrument motion, the use of robotic assistant surgical system during minimally invasive surgeries is becoming more and more common (50). In 2004, Kernstine (22) first reported the use of da Vinci surgical system during thoracic and abdominal mobilization of the esophagus during MIE and, since then, more and more surgeons (23,24,51-53) have reported robot-assisted surgery successfully. Sarkaria (23) first described its use during the Ivor Lewis—MIE, while Lehenbauer (24) first described its use during the McKeown—MIE. The ports location for robotic assisted Ivor-Lewis MIE in our hospital was shown in Figures 1,2.
Though many institutions are using robotic assistant surgical system during esophagectomy, until the results of the robot-assisted esophagectomy clinical trial are published, there is no level 1 evidence to show perioperative and long-term benefits of using robot-assisted esophagectomy. Moreover, considering the costly hospital expenses and small installation volume, robot-assisted esophagectomy will not be the main surgical approach in the next couple years.
Summary
After decades of development, thoraco-laparoscopic esophagectomy has come to an age of maturity. This surgical approach is reliable, efficiency, minimally invasive and affordable for patients. For quite a long time, it will be the main surgical approach for esophageal cancer patients.
Acknowledgments
Funding: This study was supported by the grant from National Natural Science Foundation of China (81272608), Science and Technology Commission of Shanghai Municipality (16411966100), and Shanghai Municipal Commission of Health and Family Planning (20164Y0253).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Luca Bertolaccini and Piergiorgio Solli) for the series “VATS: the age of maturity” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2016.12.03). The series “VATS: the age of maturity” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- GLOBOCAN 2012. Online. Accessed at 10.31.2016. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Torek F. The first successful case of resection of the thoracic portion of the esophagus for carcinoma. Surg Gynecol Obstet 1913;16:614.
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013;380:2095-128. [Crossref] [PubMed]
- Hur C, Miller M, Kong CY, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer 2013;119:1149-58. [Crossref] [PubMed]
- Lewis I. The surgical treatment of carcinoma of the oesophagus† with special reference to a new operation for growths of the middle third. Br J Surg 1946;34:18-31. [Crossref] [PubMed]
- Sweet RH. Transthoracic Resection of the Esophagus and Stomach for Carcinoma: Analysis of the Postoperative Complications, Causes of Death, and Late Results of Operation. Ann Surg 1945;121:272-84. [Crossref] [PubMed]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [Crossref] [PubMed]
- Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb 1992;37:7-11. [PubMed]
- Kawahara K, Maekawa T, Okabayashi K, et al. Video-assisted thoracoscopic esophagectomy for esophageal cancer. Surg Endosc 1999;13:218-23. [Crossref] [PubMed]
- Luketich JD, Schauer PR, Christie NA, et al. Minimally invasive esophagectomy. Ann Thorac Surg 2000;70:906-11; discussion 911-2. [Crossref] [PubMed]
- Luketich JD, Fernando HC, Christie NA, et al. Outcomes after minimally invasive esophagomyotomy. Ann Thorac Surg 2001;72:1909-12; discussion 1912-3.
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Ohsawa T. Surgery of the esophagus. Arch Jpn Chir 1933;10:605.
- Park SY, Kim DJ, Yu WS, et al. Robot-assisted thoracoscopic esophagectomy with extensive mediastinal lymphadenectomy: experience with 114 consecutive patients with intrathoracic esophageal cancer. Dis Esophagus 2016;29:326-32. [Crossref] [PubMed]
- McKeown KC. The surgical treatment of carcinoma of the oesophagus. Proc R Soc Med 1974;67:389-95. [PubMed]
- Skinner DB. En bloc resection for neoplasms of the esophagus and cardia. J Thorac Cardiovasc Surg 1983;85:59-71. [PubMed]
- Orringer MB, Sloan H. Esophagectomy without thoracotomy. J Thorac Cardiovasc Surg 1978;76:643-54. [PubMed]
- DePaula AL, Hashiba K, Ferreira EA, et al. Laparoscopic transhiatal esophagectomy with esophagogastroplasty. Surg Laparosc Endosc 1995;5:1-5. [PubMed]
- Kernstine KH, DeArmond DT, Karimi M, et al. The robotic, 2-stage, 3-field esophagolymphadenectomy. J Thorac Cardiovasc Surg 2004;127:1847-9. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP. Robotic-assisted minimally invasive esophagectomy: the Ivor Lewis approach. Thorac Surg Clin 2014;24:211-22. vii. [Crossref] [PubMed]
- Lehenbauer D, Kernstine KH. Robotic esophagectomy: modified McKeown approach. Thorac Surg Clin 2014;24:203-9. vii. [Crossref] [PubMed]
- Orringer MB. Technical aids in performing transhiatal esophagectomy without thoracotomy. Ann Thorac Surg 1984;38:128-32. [Crossref] [PubMed]
- Chang AC, Ji H, Birkmeyer NJ, et al. Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg 2008;85:424-9. [Crossref] [PubMed]
- Hulscher JB, Tijssen JG, Obertop H, et al. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg 2001;72:306-13. [Crossref] [PubMed]
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. [Crossref] [PubMed]
- Cuschieri A. Endoscopic subtotal oesophagectomy for cancer using the right thoracoscopic approach. Surg Oncol 1993;2:3-11. [Crossref] [PubMed]
- Cuschieri A. Thoracoscopic subtotal oesophagectomy. Endosc Surg Allied Technol 1994;2:21-5. [PubMed]
- Akaishi T, Kaneda I, Higuchi N, et al. Thoracoscopic en bloc total esophagectomy with radical mediastinal lymphadenectomy. J Thorac Cardiovasc Surg 1996;112:1533-40; discussion 1540-1. [Crossref] [PubMed]
- Dexter SP, Martin IG, McMahon MJ. Radical thoracoscopic esophagectomy for cancer. Surg Endosc 1996;10:147-51. [Crossref] [PubMed]
- Law S, Fok M, Chu KM, et al. Thoracoscopic esophagectomy for esophageal cancer. Surgery 1997;122:8-14. [Crossref] [PubMed]
- Wang Q, Wu Z, Chen G, et al. Two-Stage Indicators to Assess Learning Curves for Minimally Invasive Ivor Lewis Esophagectomy. Thorac Cardiovasc Surg 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Taguchi S, Osugi H, Higashino M, et al. Comparison of three-field esophagectomy for esophageal cancer incorporating open or thoracoscopic thoracotomy. Surg Endosc 2003;17:1445-50. [Crossref] [PubMed]
- Osugi H, Takemura M, Higashino M, et al. A comparison of video-assisted thoracoscopic oesophagectomy and radical lymph node dissection for squamous cell cancer of the oesophagus with open operation. Br J Surg 2003;90:108-13. [Crossref] [PubMed]
- Burdall OC, Boddy AP, Fullick J, et al. A comparative study of survival after minimally invasive and open oesophagectomy. Surg Endosc 2015;29:431-7. [Crossref] [PubMed]
- Smithers BM, Gotley DC, Martin I, et al. Comparison of the outcomes between open and minimally invasive esophagectomy. Ann Surg 2007;245:232-40. [Crossref] [PubMed]
- Berger AC, Bloomenthal A, Weksler B, et al. Oncologic efficacy is not compromised, and may be improved with minimally invasive esophagectomy. J Am Coll Surg 2011;212:560-6; discussion 566-8. [Crossref] [PubMed]
- Palazzo F, Rosato EL, Chaudhary A, et al. Minimally invasive esophagectomy provides significant survival advantage compared with open or hybrid esophagectomy for patients with cancers of the esophagus and gastroesophageal junction. J Am Coll Surg 2015;220:672-9. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Mamidanna R, Bottle A, Aylin P, et al. Short-term outcomes following open versus minimally invasive esophagectomy for cancer in England: a population-based national study. Ann Surg 2012;255:197-203. [Crossref] [PubMed]
- Guo W, Ma X, Yang S, et al. Combined thoracoscopic-laparoscopic esophagectomy versus open esophagectomy: a meta-analysis of outcomes. Surg Endosc 2016;30:3873-81. [Crossref] [PubMed]
- Sgourakis G, Gockel I, Radtke A, et al. Minimally invasive versus open esophagectomy: meta-analysis of outcomes. Dig Dis Sci 2010;55:3031-40. [Crossref] [PubMed]
- Nagpal K, Ahmed K, Vats A, et al. Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc 2010;24:1621-9. [Crossref] [PubMed]
- Zhou C, Ma G, Li X, et al. Is minimally invasive esophagectomy effective for preventing anastomotic leakages after esophagectomy for cancer? A systematic review and meta-analysis. World J Surg Oncol 2015;13:269. [Crossref] [PubMed]
- Biere SS, Cuesta MA, van der Peet DL. Minimally invasive versus open esophagectomy for cancer: a systematic review and meta-analysis. Minerva Chir 2009;64:121-33. [PubMed]
- Guo W, Ma L, Zhang Y, et al. Totally minimally invasive Ivor-Lewis esophagectomy with single-utility incision video-assisted thoracoscopic surgery for treatment of mid-lower esophageal cancer. Dis Esophagus 2016;29:139-45. [Crossref] [PubMed]
- Zheng Y, Li Y, Wang Z, et al. A video demonstration of the Li's anastomosis-the key part of the "non-tube no fasting" fast track program for resectable esophageal carcinoma. J Thorac Dis 2015;7:1264-8. [PubMed]
- Yamamoto M, Weber JM, Karl RC, et al. Minimally invasive surgery for esophageal cancer: review of the literature and institutional experience. Cancer Control 2013;20:130-7. [PubMed]
- Puntambekar SP, Rayate N, Joshi S, et al. Robotic transthoracic esophagectomy in the prone position: experience with 32 patients with esophageal cancer. J Thorac Cardiovasc Surg 2011;142:1283-4. [Crossref] [PubMed]
- Boone J, Borel Rinkes IH, van Hillegersberg R. Robot-assisted thoracolaparoscopic esophagolymphadenectomy for esophageal cancer. Surg Endosc 2007;21:2342-3. [Crossref] [PubMed]
- Horgan S, Berger RA, Elli EF, et al. Robotic-assisted minimally invasive transhiatal esophagectomy. Am Surg 2003;69:624-6. [PubMed]
Cite this article as: Guo W, Xiang J, Yang S, Li H. Video-assisted thoracic surgery for esophagectomy: evolution and prosperity. Video-assist Thorac Surg 2017;2:2.