
Constructing tunnels to troubleshoot complete pleural symphysis during video-assisted thoracic surgery
Introduction
The application of video-assisted thoracic surgery (VATS) has greatly increased with the improvements in endoscopic equipment and the refinement of surgical techniques. Accumulated data have shown its advantage of minimal invasiveness in the treatment of benign and malignant lung diseases (1). However, pleural symphysis (PS) is once considered a relative contraindication of VATS (2). Complete PS always prevents lung collapse at the start of thoracoscopy, increasing the risk of lung injury from the trocar or thoracoscopy, and in severe cases, prevents access to the pleural space, requiring conversion to open thoracotomy. However, appropriate methods for managing this troublesome problem via VATS have rarely been reported. A novel method of constructing tunnels to access the pleural space for troubleshooting complete PS during VATS was explored in this study.
Methods
Patients
A total of 1,163 consecutive VATS major lung resections were performed by a single thoracic surgical team from the Department of Thoracic Surgery of West China Hospital, Sichuan University between May 2006 and July 2013. Cases with severe calcified hilar lymphadenopathy, diffuse pleural thickening, and/or calcified PS showing on the computerized tomography (CT) were excluded from VATS. The other preoperative criteria for planning thoracoscopic procedure were the same as those mentioned in previous studies (3-5). The hospital records of the patients were retrospectively reviewed. A total of 62 cases with diffuse PS (more than half of pleural space) were identified during thoracoscopic exploration. In addition, 17 cases with complete PS were found, and 14 cases of which were successfully managed without conversion. The characteristics of these patients, such as diagnosis, types of resection, intraoperative complications, operation time, amount of blood loss, amount of chest drainage, chest drainage duration, postoperative complications, and length of postoperative hospital stay, were carefully recorded. Informed consent of the operation was obtained from each patient before surgery. This study was approved by the institutional review board of the hospital (ID: 2016 No. 98).
Surgical techniques
General anesthesia was administered to each patient through double-lumen endotracheal intubation. Each patient was placed in the appropriate lateral decubitus position. A 1.5 cm observation incision was made in the seventh intercostal space at the midaxillary line for the thoracoscopy. Under direct vision, we cut down to the pleura carefully. We took extreme care in entering the pleural space. When it was evident that PS was present, a finger, rather than a trocar, was inserted to perform blunt adhesiolysis. A “pocket” was made by rotating the finger, which enabled access to the pleural space for the thoracoscopy (6). If blunt adhesiolysis worked, two additional utility incisions were made: a 3 cm main utility incision was made at the anterior axillary line in the third intercostal space for the upper and middle lobes and in the fourth intercostal space for the lower lobes, and a 2 cm assistant utility incision was made in the ninth intercostal space (between the posterior axillary line and subscapular line). Same method of blunt adhesiolysis with a finger was performed through the two utility incisions, respectively. The observation incision and the assistant utility incision were attempted to be linked by constructing a tunnel in the pleural space by using fingers (Figure 1A,B). Another tunnel was constructed between the observation incision and the main utility incision by using fingers, and sometimes with the help of a sponge clamp because of a little further distance between the two incisions (Figure 1C,D,E,F). With successfully constructed tunnels (Figure 1G,H), accesses to the pleural space for the thoracoscopy and thoracoscopic instruments became into reality (Figure 1I). The construction of the two tunnels is the key point for further thoracoscopic procedures. If the tunnels were not constructed, the PS might be too dense for thoracoscopic adhesiolysis, and conversion to thoracotomy is necessitated.
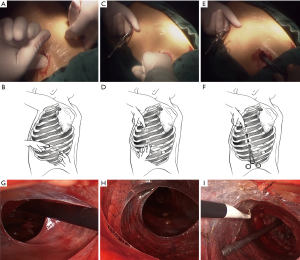
A metal endoscopic suction with side holes on its tip and an electrocoagulation hook were introduced under direct endoscopic vision into the pleural space along the two tunnels. The endoscopic suction could be used to perform blunt dissection, facilitate exposure, and immediately suck away debris and exudations to maintain a relatively clean operation field. Electrocoagulation was a basic method to dissect fibril cords and a vital option for reducing exudation. An endoscopic sponge clamp was also helpful in facilitating exposure. The alternating use of electrocoagulation and blunt dissection was effective and time saving. Moreover, dissection should always be performed closer to the parietal side to reduce pulmonary injury. The triangular pleural space between the three incisions was initially opened. After that the adhesiolysis was performed at hand. In general, the most amenable PS was taken first, and the more densely adhered PS was addressed from whichever direction it seems to be most accessible. The vagus and phrenic nerves were clearly identified and carefully protected during this procedure.
Total PS dissection was necessary for segmentectomy or lobectomy. Dissecting all of the PS was unnecessary for wedge resection and was performed only when further lobectomy was deemed necessary according to intraoperative frozen-section pathologic examination. Lobectomies were performed using the previously described single-direction lobectomy technique (3). Primary lung cancer patients underwent systemic lymph node dissection. Careful hemostasis was performed on every patient. Air leakage was meticulously checked by inflating the residual lung under pool of warm saline. Laceration sites with obvious air leakage were sutured. A chest tube was administered through the observation incision and was removed only when less than 200 mL of drainage per day, no air leakage, and complete reexpansion of the residual lung were observed. Patients without any complications were discharged from the hospital.
Results
A total of 14 cases successfully underwent adhesiolysis via VATS; 3 cases were converted to thoracotomy at the beginning of operation because the tunnels could not be constructed due to dense PS. Detailed information on the 14 patients is shown in Table 1. The median operation time was 150 min, ranging from 120 to 210 min. The median blood loss was 125 mL, ranging from 50 to 700 mL. Every patient experienced different degrees of pulmonary laceration, which caused air leakage during the operation. Lacerations with obvious air leakage were carefully sutured. The median chest drainage volume was 725 mL, ranging from 400 to 2,000 mL. The median chest drainage duration was 4 days, ranging from 2 to 12 days. Four patients suffered postoperative complications. Two cases suffered from pneumonia, and two cases suffered from prolonged air leakage (>7 days). Prolonged chest drainage and pleurodesis were performed by repeatedly administering 50% glucose solution into the pleural space to handle the prolonged air leakage. The chest tubes of the two patients were removed at the 11th and 12th postoperative days, respectively. The four patients were finally discharged from the hospital without any complications. The median length of postoperative hospital stay was 6.5 days, ranging from 4 to 18 days. Perioperative mortality was not observed.
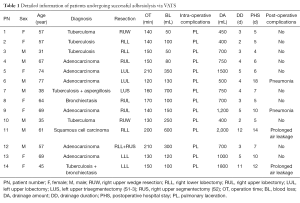
Full table
Discussion
PS is a common impediment to VATS. Loose, membranous, or cord-like adhesions are easily dissociated (6,7), but some specific strategies are required for cases with complete PS. To the best of our knowledge, detailed strategies for dealing with complete PS during VATS have not been reported in English literature. In this study, we’d like to share our experience in resolving complete PS during VATS, which might be encountered by almost every thoracic surgeon, and to discuss the indication of thoracoscopic adhesiolysis in such situation.
The key point to proceed with further thoracoscopic adhesiolysis is the initiation of accesses to the pleural space. Only if the so called tunnels were constructed could we insert thoracoscopy and instruments to proceed with further adhesiolysis. If not, conversion to thoracotomy was necessitated. Rough or forced dissection should be avoided. Three cases underwent conversion to thoracotomy because the tunnels could not be constructed through blunt dissection. In our findings advantages of thoracoscopic adhesiolysis were identified: (I) A 30° thoracoscopy provided a greater range of views and a better exposure of the blind spots such as the apical or costophrenic area of the pleural cavity than open thoracotomy; (II) the effect of thoracoscopic amplification ensured more accurate identification of regional anatomies and clearer clearance between the visceral and parietal pleura; (III) intended dissection closer to the parietal side minimized pulmonary injury; (IV) alternating use of electrocoagulation and blunt dissection according to different situations could save operation time as possible; (V) the combination of suction and electrocoagulation made adhesiolysis more vivid and safer. Instruments inserted from the two utility incisions could be more effectively combined to perform adhesiolysis if the area between the three incisions is opened beforehand. The hilum should be gradually approached from different directions.
The most common complications were lung injuries, which are sometimes inevitable because of the indistinct boundary between visceral and parietal pleura. Air leakage must be meticulously checked, and repair is needed. Prolonged postoperative effusion and air leakage are important concerns to patients with complete PS. Two cases suffered from prolonged air leakage and accompanied massive effusion in this study, and both were treated faultlessly. The operation time, blood loss, amount of chest drainage, duration of chest drainage, postoperative complications, and the length of postoperative hospital stay were acceptable.
PS is not often anticipated prior to operation. Cases with severe calcified hilar lymphadenopathy, pleural thickening, and/or calcified PS showing on CT scans were excluded before referring patients to VATS, but three cases still had unanticipated dense PS, for which the pleural space cannot be accessed. Predicting the presence of PS, their positions, and their diffusion can help establish the initial port site or choose between an open or VATS approach (8). CT scans have been reported to have limited accuracy in predicting PS (9). However, transthoracic ultrasound has been reported to be useful in PS detection before surgery (8,10,11). This novel technique will be attempted in future studies to improve the operation plan.
In conclusion, constructing tunnels in the pleural space is a novel approach to troubleshoot complete PS via VATS. The successful tunnel construction in the pleural space indicates further thoracoscopic adhesiolysis in cases with complete PS.
Acknowledgments
Funding: This paper was supported by the Key Science and Technology Program of Sichuan Province, People’s Republic of China (No. 2013SZ0005 and No. 2014SZ0148, both to Dr. Lunxu LIU).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2016.09.04). Liu L serves as the Editor-in-Chief of Video-Assisted Thoracic Surgery. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent of the operation was obtained from each patient before surgery. This study was approved by the institutional review board of the hospital (ID: 2016 No. 98).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Solaini L, Prusciano F, Bagioni P, et al. Video-assisted thoracic surgery (VATS) of the lung: analysis of intraoperative and postoperative complications over 15 years and review of the literature. Surg Endosc 2008;22:298-310. [Crossref] [PubMed]
- Doddoli C, Barlési F, Fraticelli A, et al. Video-assisted thoracoscopic management of recurrent primary spontaneous pneumothorax after prior talc pleurodesis: a feasible, safe and efficient treatment option. Eur J Cardiothorac Surg 2004;26:889-92. [Crossref] [PubMed]
- Liu L, Che G, Pu Q, et al. A new concept of endoscopic lung cancer resection: Single-direction thoracoscopic lobectomy. Surg Oncol 2010;19:e71-7. [Crossref] [PubMed]
- Mei J, Pu Q, Liao H, et al. A novel method for troubleshooting vascular injury during anatomic thoracoscopic pulmonary resection without conversion to thoracotomy. Surg Endosc 2013;27:530-7. [Crossref] [PubMed]
- Pu Q, Liu LX, Che GW, et al. The feasibility study in the treatment of benign pulmonary diseases by single-direction complete video-assisted thoracoscopic lobectomy. Sichuan Da Xue Xue Bao Yi Xue Ban 2010;41:548-50. [PubMed]
- Demmy TL, James TA, Swanson SJ, et al. Troubleshooting video-assisted thoracic surgery lobectomy. Ann Thorac Surg 2005;79:1744-52; discussion 1753.
- Li Y, Wang J, Yang F, et al. Indications for conversion of thoracoscopic to open thoracotomy in video-assisted thoracoscopic lobectomy. ANZ J Surg 2012;82:245-50. [Crossref] [PubMed]
- Wei B, Wang T, Jiang F, et al. Use of transthoracic ultrasound to predict pleural adhesions: a prospective blinded study. Thorac Cardiovasc Surg 2012;60:101-4. [Crossref] [PubMed]
- Mason AC, Miller BH, Krasna MJ, et al. Accuracy of CT for the detection of pleural adhesions: correlation with video-assisted thoracoscopic surgery. Chest 1999;115:423-7. [Crossref] [PubMed]
- Cassanelli N, Caroli G, Dolci G, et al. Accuracy of transthoracic ultrasound for the detection of pleural adhesions. Eur J Cardiothorac Surg 2012;42:813-8; discussion 818. [Crossref] [PubMed]
- Sasaki M, Kawabe M, Hirai S, et al. Preoperative detection of pleural adhesions by chest ultrasonography. Ann Thorac Surg 2005;80:439-42. [Crossref] [PubMed]
Cite this article as: Liu C, Pu Q, Liao H, Ma L, Mei J, Lin F, Guo C, Liu L. Constructing tunnels to troubleshoot complete pleural symphysis during video-assisted thoracic surgery. Video-assist Thorac Surg 2016;1:23.


